Spectroscopy Series VOL. 15: Mass Spectroscopy
The science is advancing rapidly every day without even realizing it and we can say that more and more it is more complicated to be able to assimilate so much information, with this I mean new concepts that involve these new inventions, however, to all this, scientists must face it and adapt to these technologies that help development.
The spectroscopic techniques have played a fundamental role in science for a long time and in my personal opinion any professional should have knowledge of them, since they cover practically all scientific areas and subareas.
Mass spectroscopy is one of the oldest, however, it is still used today despite the advances that have been made in recent decades with the creation of new highly sophisticated and easy to use spectrometers. Next, I will talk about some important aspects on which this technique is based, both theoretical and experimental. I hope you like it.

To begin to explain this fabulous spectroscopy technique we must know what it means. It is an analysis technique that allows to analyze different materials or compounds of organic, inorganic and biological nature. We can obtain both qualitative and quantitative information. The EDM allows to determine the distribution of the molecules of different materials and to be a substance always based on its mass. It also has the possibility of obtaining very effective information of the molecular mass of the analyzed material and from here it is possible to extract information from the atomic structure of this, with only detecting its presence or quantifying its chemical concentration.
Something we must take into account is that mass spectrometry is not considered a spectroscopic method, although many scientists claim the opposite. One big difference between mass spectroscopy and other classical spectroscopic techniques is that, if we focus on obtaining the spectrum from the classical point of view, this provides us with two-dimensional information that brings as a final result parameters obtained due to the emission or absorption that is made to the material. It is a very complex issue because also another notable difference is that for the classic spectroscopic techniques the physical processes that are carried out to the samples to then obtain the spectrum does not need to be modified chemically and therefore the sample can be reusable for other measurements , in contrast, in mass spectroscopy, the sample is completely lost after being scanned, because the amount needed to obtain a spectra is minimal and very negligible.
The physical basis of this technique is focused on obtaining ions from different molecules within the material that is to be analyzed. These molecules are mostly organic in phase of gaseous state that move in the presence of a magnetic field after obtaining the ions. of these molecules within the material we proceed to separate their mass and elemental charge, where finally and thanks to a specialized detection equipment we can observe the spectrum.
Then a mass spectrum, gives us two-dimensional information of the ionic phenomenon involved in this process, which is represented by a process that involves certain parameters of these ions that give precise information as a function of the mass and charge of the material.
A very powerful and precise tool, since through mass spectroscopy we can obtain information about the structure of quite complex molecular samples to analyze by other techniques and according to the qualitative and quantitative analysis we can determine its chemical composition through the spectra of masses.
We know that the spectrometer used for this experimental analysis technique measures mass / charge ratios of ions, by heating precisely one beam of the sample of the compound that we want to analyze until we can finally vaporize and ionize the atoms inside the sample.
The ion beam produces a specific pattern in the detector, which allows the compound to be analyzed. In the industry it is a highly used technique in the elemental analysis of semiconductors, biosensors, complex polymer chains, drugs, chemical synthesis products, forensic analysis, environmental contamination, perfumes and all types of analytes that are susceptible to pass to vapor phase and ionize without decomposing.
The processes involved in the mass spectrometer are of a chemical nature, that is, the information collected from the materials by means of the spectra of certain types of ions that can always be identified in the material through its mass, are obtained through of its chemical structure, the information extracted from these spectra are compared in some way or another by means of a quantity of chemical reactions for the determination of said chemical structures, then this equipment is a very powerful tool that can offer a lot of information of different compounds.
JJ Thomson in 1912 when the scientist pushed by his desire to discover the deepest secrets of chemistry, he managed to create the first mass spectrometer and get the first spectra of elements such as O2, N2, CO and COCl2 .
Next, we will focus on talking about the fundamentals of the mass spectrometer that through the following scheme below, we will explain step by step each of them.
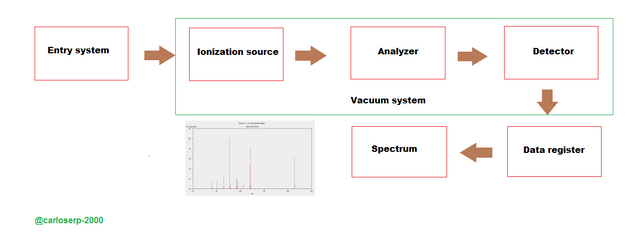
Diagram of a mass spectrometer
This team must be able to perform certain essential tasks for its perfect operation which are:
Must have the ability to vaporize highly volatile substances, and in turn must have the ability to create ions from molecules in a gaseous state. After passing this stage and having generated the ions, it must fulfill the most important task that is to separate the ions according to the mass / charge ratio of the material. And finally, after vaporizing the ions and separating them from the previous relationship, detecting and forming them should record the information and translate it into the mass spectrum.
Below I will explain each stage of the process involved in mass spectroscopy:
The entry system: Its main objective is to introduce a certain amount of sample of the material in the spectrometer, this amount must be very small in the order of a micro or perhaps less. One of the main problems presented by this spectroscopy technique is the limitation of being able to vaporize the sample, so that a spectrum can be obtained that can be visualized perfectly, an approximate vapor pressure of 10exp-6 mm of mercury is needed, it should be noted that it is not necessary to completely evaporate the sample but only a portion that can reach the pressure previously indicated.
3 methods of sample introduction are used which are: Direct, indirect and of a chromatograph.
The first is to introduce the sample of the material that you want to analyze directly in the ionization source, this is done carefully with a kind of metal tube, where the sample is held at the tip. The next step is to heat the sample directly using a variable resistance, it should be noted that the sample should not be left to hold the tube, then proceed to introduce it in the ion source, where it is completely empty, said vacuum is regulated by different valves to not let himself escape.
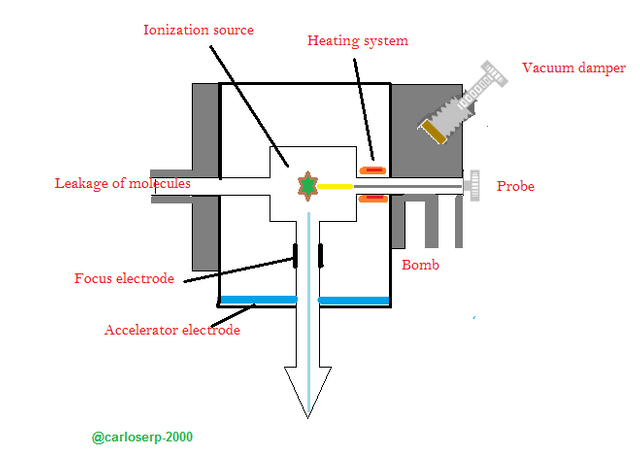
Schematic of the input system of a sample in the mass spectrometer
The second is based on vaporizing the sample outside the measuring equipment, it is advisable to use a fully enameled glass container inside to help maintain the indicated temperature, that is, the enamel acts as a powerful thermal insulator. Afterwards, the sample must be introduced carefully, it should be noted that for this type of spectroscopic technique the samples are very small and especially the metal ones are so fine that they can be broken, so to avoid catalysis processes that can alter the sample, a careful handling. This method can also be applied in gases and liquids with a boiling point of approximately 200º C, it is recommended to have a container with a capacity of approximately 1 liter.
After the material is vaporized inside the container at an approximate pressure of 10exp-2 mm of mercury, it is let out in the vapor gas towards the ion source, due to the high pressures this gas flows very fast which allows the molecules can pass to the source of ions at a constant rate.
And finally the method of the chromatograph is undoubtedly one of the most used now since no preparation is needed for the sample, simply must be introduced directly, whether liquid, solid or gas phase.
Ion source is in charge of converting the sample into ions already by means of the bombardment of electrons or photons towards the material. Another option that is used a lot for the transformation of ions is through thermal or electrical energy.
The ionization techniques have been fundamental to determine which types of samples can be analyzed by mass spectrometry. The ionization of the electron and the molecular ionization are used for gases and vapors.
There are a variety of ionization sources, including gas, where the sample is first volatilized and then ionized. Another is the source of desorption, where the ions to reach the gaseous state first need to inject energy directly from solid and liquid phases.
Ionization by means of electronic impact is another method where the samples are ionized through the bombardment of electrons at a very high energy. In order to originate the ionization, electrons are used with an incandescent filament very similar to that of a light bulb, these emit a thermoelectric energy and are accelerated due to the difference of voltage variable potential.
Another important characteristic of this ionization method is that a parallel magnetic field should be used in the direction of the electron path, this in order to be able to focus correctly and can describe a helical path until finally reaching the anode.
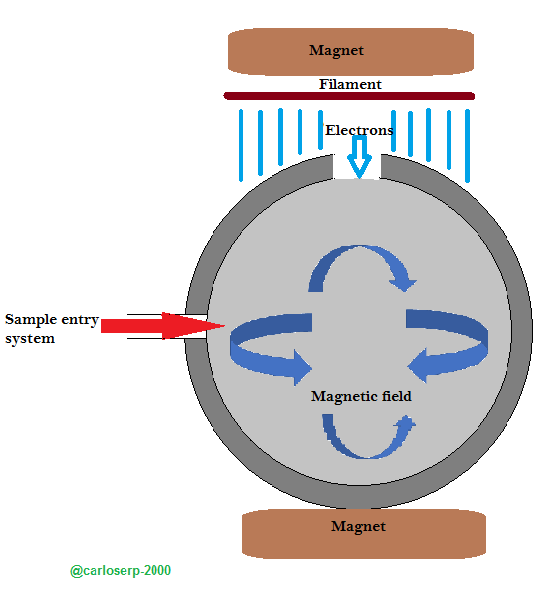
Diagram of the method of ionization by electronic impact
Another method is chemical ionization, in which an ionizing material is normally used that can convert the ion and transfer its charge to the molecules of the material due to a molecular chemical reaction.
We must introduce a methane in the source of ionization and produce a determined pressure around 1 to 1.5 mm of mercury, so that the reaction can take place, thanks to the fact that the electrons essentially ionize the methane molecules within the sample.
There are also two more types of ionization sources and these are applied according to the type of sample whether hard or soft.
In hard sources the ions are in vibrational and rotational states so it has very high energies to the ions that can be formed, when these ions relax they tend to fragment, which translates into the creation of very complex unique mass spectra.
In contrast, soft sources are the opposite of the previous one, in this the excitation produced in the ions produces a fragmentation that gives rise to quite simple spectra and easy to analyze.
And this is all for this for this occasion, coming soon next I will continue talking about this interesting topic.
You want to know more about my spectroscopy series visit the following links:
Vol.1 Vol.2 Vol.3 Vol.4 Vol.5 Vol.6 Vol.7 Vol.8 Vol.9 Vol.10 Vol.11 Vol.12 Vol.13 Vol.14If you want more information about the subject you can visit the following links:
Mass Spectrometry/ Prem,ier Biosoft
Analytical chemistry/ New world enciclopedy
How Does Mass Spectroscopy Work?
Plasma Atomic Emission Spectroscopy
Chemical ionization/ Wikipedia
The Ionization of Atoms by Electron Impact
Publish through our official app and you will get an extra vote of 5% https://www.steemstem.io/
 Video credits @gtg
Video credits @gtg
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and utopian-io!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Hi @carloserp-2000!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 20 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 6 SBD worth and should receive 205 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePig