Spectroscopy Series VOL. 10: XPS Spectroscopy
Welcome again to another installment of my spectroscopy series, where I will show you a very powerful technique that we use to determine the chemical composition of surfaces of different materials. Due to its variety of applications, it is widely used in different industries worldwide.
But before starting the content of this publication I suggest you visit my previous deliveries:
One of the most used techniques for the chemical analysis of surfaces of materials is the photoelectronic spectroscopy of X-rays or electronic spectroscopy of chemical analysis. Its physical foundation lies in the interaction of a beam of photons with matter.
In general, it can be said that this spectroscopic technique is ideal for analyzing any material chemically, because it provides information about its atomic composition, electronic structure, oxidation state and stoichiometric estimation of different elements that can be examined through it.
X-ray spectroscopy is also known by many as ESCA (electronic spectroscopy for chemical analysis). Where the energy of a photon impinges on atoms located on the surfaces of the compounds to be analyzed and results in the emission of photoelectrons with a binding energy: EB = hυ - EK - W. Where hv is the energy of the photons, EK, the kinetic energy of the produced photoelectron, W, the working function of the spectrometer and EB, the binding energy.
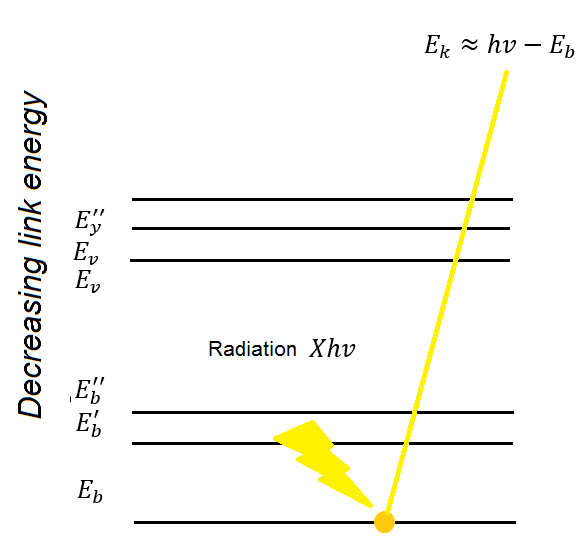
Diagram of the X-ray photoelectron spectroscopy process, showing the 3 lowest lines that represent the energies of the electrons of the layers of the atom and others represent the energy levels of the valence layer.
Let's talk about The physical principle of this technique.
It consists in the irradiation of a beam of X-rays that later hits the sample by exciting the electrons of the atoms, and if the energy with which the atoms are bound is less than the energy of the X-rays, the electrons can jump from the orbital in form of photoelectron, is therefore the name that carries the technique. Subsequently, most of the electrons are reabsorbed by the sample except for that which is produced near the surface of the sample (depth of 10 to 100 Armstrong).
After analyzing the energy of the photoelectrons and having exact knowledge of the energy of the incident X-rays, we can obtain valuable information about the elemental composition of any material that we wish to analyze.
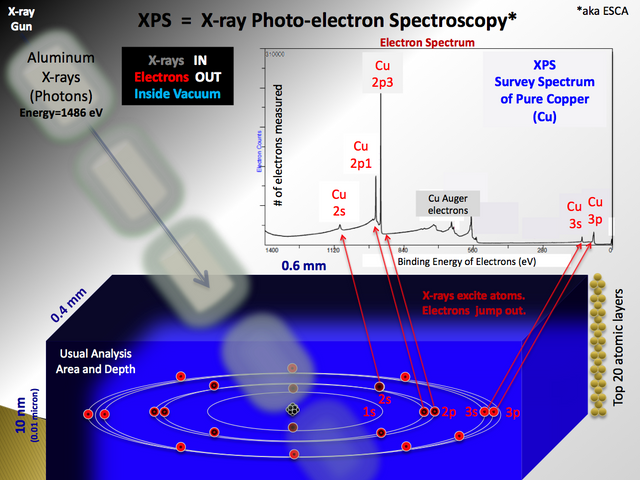
This schematic depicts the process known as the "Photo-electric Effect" as it pertains to XPS. Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0)
Now we will focus completely on talking experimentally about an X-ray photoelectron spectrometer, in turn taking into consideration the important elements for its proper functioning, indispensable for characterizing any type of material.
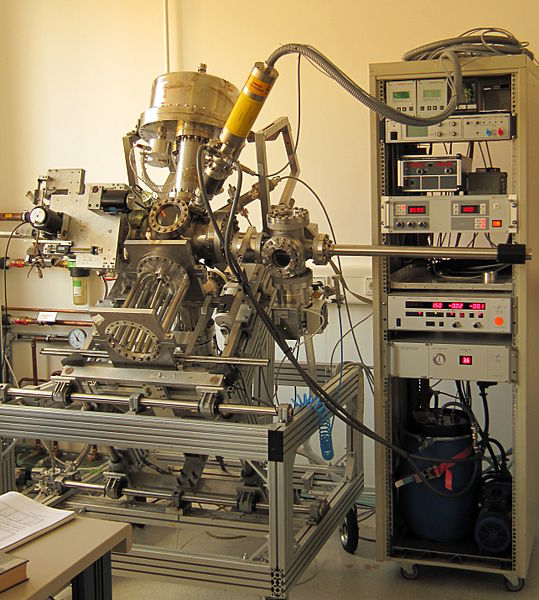
XPS spectrometer Atribución 3.0 Unported (CC BY 3.0)
An XPS spectrometer is composed of the following components:
A sample injector arm, whose main function is to load and inject the sample into the chamber of the slide.
The vacuum pre-chamber and sample parking, as its name indicates, serves to house various materials that are to be analyzed in the sample holder at the same time in vacuum-controlled conditions.
It consists of an electron analyzer, which allows you to filter your electrons by energy.
Next of an electron detector or signal, whose signal comes from the analyzer.
A source of monochromatic light beam, which emit electromagnetic radiation with high intensity.
Focused spot, which is used for the analysis in discrete areas of the samples with a high spectral resolution.
A non-monochromatic light source serves to irradiate the entire surface of the analyzed sample to obtain a quantitative analysis of the chemical composition in heterogeneous materials.
Ion cannon, used for cleaning and depth profiles of samples.
Optical system of visualization, where we observe the image of the surface of the sample.
And finally the Ultra Vacuum Chamber.
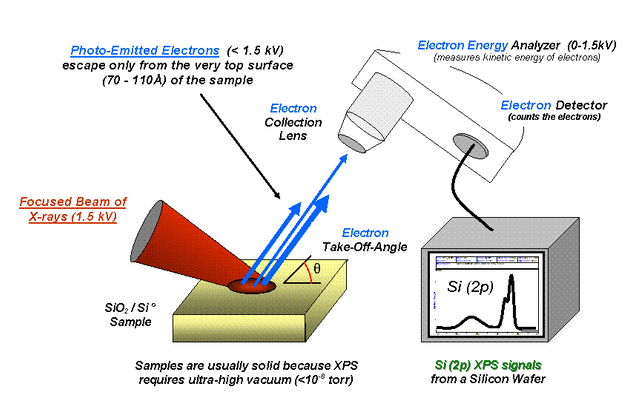
Diagram of the principle of X-ray photoelectron spectroscopy Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0)
We already talked about the basic principle of XPS spectroscopy and we also explained each component of the equipment used to analyze materials, after all this we went to the next stage where I will explain the proper procedure to perform XPS measurements.
First of all, there is a sample that is placed in the holder of the sample holder, to introduce it we must open the vacuum chamber gate, for this the existing vacuum must be broken by stopping the pumping system introducing an inert gas, a Once the atmospheric pressure is reached, the pre-chamber gate opens and then we place the sample with the support inside, holding it with a click system on the injector arm. The system is closed and the pumping must be restarted so that the pre-chamber reaches the level of adequate vacuum that allows the passage of the sample holder inside the main analysis chamber. Once the vacuum level in the prechamber has been reached, the main valve that separates the two chambers must be opened and through the injector arm the sample is introduced into the main analysis chamber and the vacuum stabilization is expected through a system Positioning is adjusted in the area that we want to measure, then the ion cannon is started and we check that the maximum intensity of photoelectrons is obtained in the area indicated by us. Finally, in the software we will give the order to start the measurement, obtaining the spectra for the elemental quantification and the high resolution spectra in order to determine the chemical environment.
Let's explain what the XPS spectrum means.
This vse is recognized as having a series of peaks that correspond to the binding energies of the detected photoelectrons, measuring the area under each peak and multiplying by sensitivity factors of each type of atom the quantitative analysis of the chemical composition of the sample is performed . The XPS not only gives us elementary information about the sample, but it can also give important information about the chemical environment of the sample, since depending on the atoms to which they are linked to the parent atom, the energies of the photoelectrons can change. The instrument is sensitive enough to detect small variations of binding energies, which allows determining which chemical compounds are present in the material.
Important: In this technique by means of the ion cannon we can obtain a profile at depth of the composition of the sample, by removing the material layer by layer following the photoelectrons.
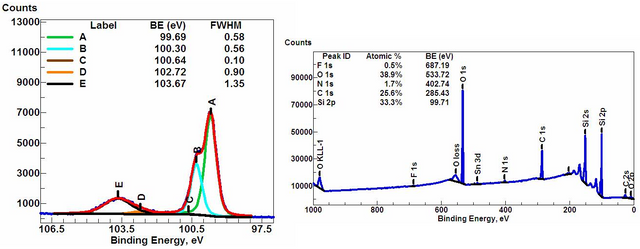
Some examples of the XPS spectra. On the left a spectrum "High Energy Resolution XPS Spectrum" and the right "Wide exploration scan spectrum". Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0) Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0)
Some of the applications of the XPS technique.
In the metallurgy with regard to the segregation in the grain limit with the growth of crystals and solidification process of inhomogeneous materials, electronic structure and alloys, composition of surfaces of different materials and quality controls.
Surface engineering: tests on wear resistance, corrosion resistance, coating of samples and interaction with other surfaces.
Corrosion phenomena, showing the interaction of the surface with the environment and surface breakage of the layer by localized phenomena.
Heterogeneous catalysis, where techniques are used for the characterization of surfaces in different materials that gives us information about the chemical environment, dispersion, oxidation state and elemental composition of the surfaces of the materials.
In microelectronics with regard to the increase in the density of transistors with high operating frequencies.
In polymer materials in the structural characterization of different materials, it shows information of its chemical composition.
In adhesion phenomena, applied in different industries such as footwear, furniture, automobiles, aeronautics. In order to determine if there is a contaminant on the surface of an adhesive foam and / or on the painted side of automobile panels, with this we can identify what type of contaminant is in the paint.
Conclusion.
> This technique allows us to chemically analyze the surfaces of different materials and the chemical environment of each of the elements that compose it. Importantly, if we make correct use of the ion beam we can remove monolayer to monolayer to obtain an in-depth analysis of the elements that are present within the material. For this reason this technique is one of the most powerful and most used today thanks to the number of applications it has and its effectiveness since the main advantage is that it corresponds to a non-destructive chemical analysis of different materials.
If you want more information about the subject you can visit the following links:
X-ray photoelectron spectroscopy
X-Ray Photoelectron Spectroscopy
X-ray Photoelectron Spectroscopy (XPS)

Video credits @gtg
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and utopian-io!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Hi @carloserp-2000!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV