Theoretical compilation of the raman effect (introduction to spectroscopy)
In this post I will talk a little about one of the oldest techniques of physics, which today have served as the basis for many other spectroscopic techniques and through it have generated many studies that have now been applied in different areas of science.
Below I will show a theoretical compilation of Raman spectroscopy, primordial points that we must know to understand a bit about this technique.
Raman spectroscopy is a technique widely used in many fields of science, as I just mentioned, which serves to analyze different materials, whether they are solids, liquid substances, gaseous substances, among others. I can say that the result of spectroscopy that is done experimentally to any specimen, you get a fingerprint or identification card (this I can say in a simple way so that readers can grasp the idea of what I'm writing). What makes this technique different from other techniques, is that Raman spectroscopy is performed directly on the material without the need to prepare the sample before conducting the experiment, it is not necessary to submit the sample to a chemical or physical treatment.
Maybe I can say that the main use of this technique is to determine the modes of vibration of the crystal lattice, that is, the low frequency modes, also the rotation modes, all this gives us important information about the structure of a material. An application to the solid state physics in which I develop and have more knowledge.
When we talk about the modes of vibration of the Christian network we have to talk about crystal structure, phonons, etc., it is somewhat complicated, but today we have different programs and measurement instruments to facilitate the analysis of this type of studies. It is likely that in the future I can write a post on this topic as it is quite extensive, in this writing I will focus only on talking about a small introduction of Raman.
We can use Raman spectroscopy for many things, both for qualitative and quantitative applications. The characteristic spectra of Raman are very specific and the chemical results can be done through the use of search algorithms in digital databases of different computer programs. As in infrared spectroscopy, the band areas are proportional to the chemical concentration of specific material, which results in this technique being susceptible to quantitative analysis. In fact, because Raman bands are intrinsically sharper than their infrared counterparts, isolated bands are often present in the spectrum for more direct quantitative analysis. This is great!!!
The effect of Raman is produced through a photon which penetrates a molecule, which in turn interacts with many electrons bound in the molecule of the material to be characterized. As a result, the photon that falls on this molecule causes as a consequence that it moves from one side to another generating that it moves to a virtual state.
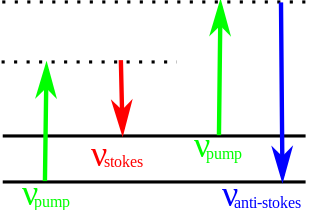
Diagram of energy levels for Stokes and anti-Stokes change present in optical effects such as Rayleigh, Raman and Brillouin scattering licencia Creative Commons Attribution-Share Alike 3.0
Basically the molecule is excited from a first state called "fundamental" to a state of "virtual energy" and is relaxed to an excited vibrational state, which generates the so-called Raman Stokes scattering. If the molecule was already in an excited vibrational state, the Raman scattering is called Raman anti-Stokes scattering.
This effect uses a monochromatic laser light source to influence the sample in order to generate a spectrum where the Raman effect can be visualized, using a detector camera. This light generates a characteristic spectral pattern of a material and allows in turn to identify substances that evaluate and determine the vibrations, the crystallinity and the orientation of the low frequency modes.

Bust of Chandrasekhara Venkata Raman (1888-1970) licencia Creative Commons Attribution-Share Alike
The name of this effect is due to its creator Sir Chandrasekhara Venkata Raman One of the most copnocidan names in the scientific field, was a respected Indian physicist who gave a very important contribution to science that today is used for different applications , his main contribution as we all know is in the area of physics. In 1928, together with his colleague, KS Krishman discovered the wonderful Raman effect, which affirmed the quantum nature of light. The following year he was appointed by the British crown Sir. He received the Nobel Prize in Physics in 1930 for the discovery that when light passes through a transparent material, part of the diffracted light changes its wavelength. This phenomenon is currently known as Raman scattering and is the result of the Raman effect. In addition, in 1932, he discovered the spin of the quantum photon together with the Bhagavantam.
After its discovery, the Raman effect became the preferred technique for many people dedicated to science for the analysis of infrared absorption spectroscopy, although it should be noted that at that time the technique, although very effective for such analyzes, was complex and needed trained personnel to calibrate and assemble the equipment for its subsequent characterization of materials, this staff had to use very consistent equipment in mercury lamps, spectrographs or photographic films to obtain the spectra.
Over the years this technique was perfected, I could say that it became the best and in 1960 was very close to experiencing great progress with the appearance of the laser that replaced the mercury lamp and later the development of fiber optic In the 80s, the technique was acquiring adequate perfection and the spectra were observed with higher quality and sharpness. Later, in the 90s, filters, gratings or diffraction gratings and coupled charge detectors were created, converting this spectroscopy into the best current technology, the most powerful and easy to use. We must bear in mind that at present we have available portable equipment easy to handle and assemble.
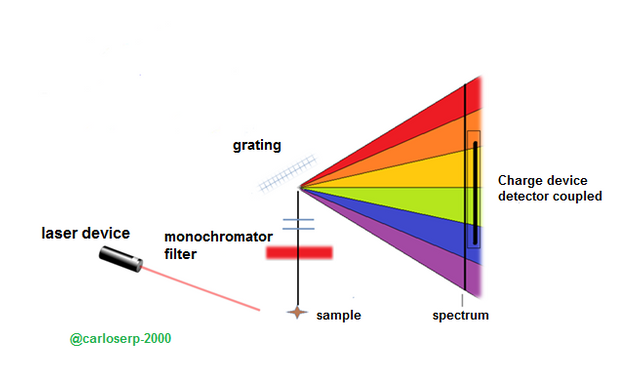
Diagram of the dispersive energy Raman
When we visualize a characteristic raman spectrum we can observe three types of dispersion. The first is the Rayleigh line, it is an electromagnetic radiation that falls on the molecule inside the material, this is the beginning of the transition to the next level or scale, when it loses the radiation this molecule must return to the start state, which means that no change in energy is observed and, that is, the incident and scattered radiation have equal wavelength and frequency.
A process quite similar to the Rayleigh lines, are also called Stokes lines, but there is a small difference between the two, the molecule tends to lose excitation and remains in the state of vibration and results in the absorption of the length of wave and dispersion of the other level.
And the third are the Anti-Stokes lines, they are basically the same as the Stokes lines, unlike the molecule returns to the lowest level and brings as immediate consequence that the scattered radiation is greater than the absorbed and this translates into a much higher frequency .
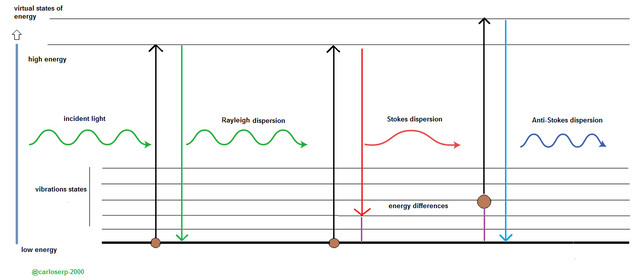
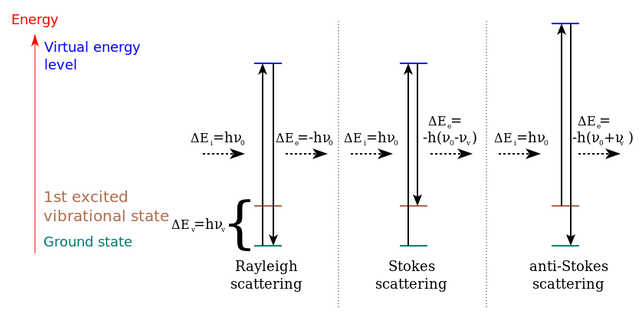
Diagram of energy levels of the states in the Raman dispersion
We can say that the raman effect basically consists of obtaining a monochromatic beam of light, when this beam hits the sample, a portion of the light is scattered and has the same frequency as the wave that hits the material. Another portion of the light disperses inelastically and returns the frequency of each molecule that makes up the material, this phenomenon is known as the Raman effect.
We can observe in this phenomenon that the variations in the frequency are caused by variations of the energy between the bonds of the molecules, each link could be said to be a door that unites both masses, that when excited by the beam of light produces vibrations movements and rotation at a specific frequency of each link. That is why each of the movements of the molecules corresponds to a given energy value. The type of inelastic dispersion is different in both cases, however, if the scattered photon has a lower energy than the incidence, the Stokes dispersion occurs, and on the contrary, if the energy is greater, the dispersion of Anti-Stokes.
CC BY-SA 3.0
The different possibilities of scattering of light: Rayleigh scattering (without energy exchange: incident and scattered photons have the same energy), Stokes Raman scattering (the atom or molecule absorbs energy: the scattered photon has less energy than the incident photon) and the anti-Stokes Raman scattering (the atom or the molecule loses energy: the scattered photon has more energy than the incident photon).
to be continue.....
If you want more information about the subject you can visit the following links:

Video credits @gtg
Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 13 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 6 SBD worth and should receive 116 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePigThis post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and utopian-io!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Hi @carloserp-2000!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Excellent post @carloserp-2000. Regards.
Thanks my dear @tsoldovieri