Spectroscopy Series VOL. 12: AES Atomic Emission Spectroscopy
Hello friends, welcome again to another installment of my spectroscopy series.

But before starting the content of this publication I suggest you visit my previous deliveries:
Vol.1 Vol.2 Vol.3 Vol.4 Vol.5 Vol.6 Vol.7 Vol.8 Vol.9 Vol.10 Vol.11It is an analytical method that is based on the radiant energy emitted by atoms of an element that are in a certain state of matter. But you will wonder what this is for? What are the applications? Undoubtedly, these are the most important questions, because perhaps many people can read some scientific content related to a team and the first thing that comes to mind for what this serves?.
To begin we can say that this experimental technique is used for qualitative analysis of any material, for example, this type of analysis can be used in the determination of potassium in a certain sample of liquor such as wine, also the percentage of calcium in milk, elements present within the water, among many more.
Next, in this publication, I will focus on talking a little about what the experimental process is like to obtain atomic emission spectra. But first I must start by writing about the theory involved in this process.
So, we can say that the AES is an experimental technique used for the chemical analysis of various materials, this method analyzes the different wavelengths of a photon emitted by the atoms or molecules within the material creating a transition of energetic levels, that is to say of an excited level at a lower energy level. The radiation that is emitted due to the photon is the study that is carried out in the whole region of the spectrum. Subsequently absorbing energy they are excited and remain at an energy level for a very short period of time. When the molecule is returned to its ground state, it emits the excess energy in the form of light. All this process is caused when the sample is subjected to a large discharge of electricity supplied by a source.
Each element, compound, substance or any particular material has a characteristic spectrum that identifies it, as I mentioned in previous publications, the spectrum is the fingerprint of a material. This analyzed material presents a characteristic set of discrete wavelengths as a function of its electronic structure, that is, the emission of photons generates this particular set. Thanks to this study we can observe these wavelengths and from them be able to determine the chemical composition of the material analyzed.
We must remember that the emission spectroscopy of development during the 90's perfected dramatically to this day, all these efforts by scientists to explain the spectra led to quantum mechanics.
There are different ways in which the molecules within the analyzed material or atoms can reach a level of excitation, it is here where the first and simplest method of emission is created, which consists of heating the material to a very high temperature, for this reason the Immediate consequence is that atoms are excited, this method is known as Atomic emission spectroscopy by flame.
Anders Ångström discovered the discrete emission lines in the year 1850. The flame emission spectroscopy is based on this discovery.
In a certain way we know that these emission lines are caused by a transition of energy levels that are quantized, it is clear that we can not see this at first sight since they have a very finite width, it means that they are composed of more than one length of wave of light.
The emission lines in the hot gases were discovered by Ångström, and the technique was developed by David Alter, Gustav Kirchhoff and Robert Bunsen.
Instruments used in atomic emission spectroscopy
Basically the same components are used as in the atomic absorption spectroscopy and the principle of foundation is the same, the only difference is that different experimental methods are used for the analysis of the samples. In the first instance we must have a source of excitation with high temperatures. Similarly, the samples must use a nebulizer and then pass to the source of emission through which a gas flows, in this case it would be the liquid samples.
In the solid samples it can be incorporated in the spectrometer through an excitation source, when the monochromatic light source (laser) irradiates the sample, these must be in a suspension or ablation device, where the gas stream flows through the sample. In the same way these samples could be vaporized and excited by means of an electrode that generates a small spark of current thanks to the laser light. Then follow the different stages desolvatación, atomization by means of its components.
Also a dispersive system that is responsible for measuring the radiation emitted by the atoms or molecules of the sample analyzed. And a detection system whose job is to detect the atomic radiation of the sample and then send it to a specialized software where we can perform the spectral analysis.
Some experimental methods used in atomic emission spectroscopy
Emission by flame
In this method as its name indicates, a flame is the main component for this type of study since it is the source of emission, in this case it must provide a very high temperature so that the sample has the capacity to allow the molecules and ions can interact within the solute and then can be dispersed, this is known as desolvation, you must also have free atoms so that you can analyze the spectrum by means of vaporization.
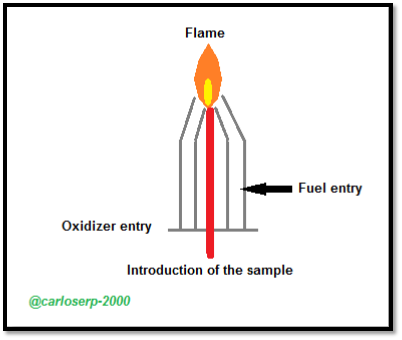
Schematic of a burner used in the flame method where the solution is completely sucked by the flame. Designed specifically for the AES, the entire process occurs inside the flame desolvation, atomization and excitation
Different systems are designed so that the fogging process is formed before it reaches the burner, ie the fuel and the oxide are mixed and interact before the flame process in the burner.
A small noticeable difference between atomic absorption spectroscopy and atomic emission spectroscopy is that in the first the atoms must be in the ground state, instead the emission must excite these atoms at higher energy levels.
Inductively coupled plasma
This method uses the following components: concentric tubes made of quartz, a radiofrequency coil that generates an induction that can be cooled with water and a Tesla coil that generates a small ionization spark of argon.
First we must know exactly what the plasma means. It is a gaseous mixture that has the property of conducting electrical current and this is because it has a considerable amount of electrons and cations. The conductive materials contain argon ions and electrons with a lower percentage of cations. When the argon ions are formed they have the wonderful ability to absorb the energy of a source and thus be able to maintain quite high temperatures.
The inductively coupled plasma method has an excitation source of a temperature that varies approximately between 8000 and 10000K. This desolvated, vaporizes and in turn excites the atoms or molecules of materials with impressive efficiency.
To summarize, the technique requires a sample of certain material where it must first be nebulized, then it is dragged through a gas stream with an Argon support. Subsequently the upper part of the plasma called torch, which is composed of concentric quartz tubes, in turn contains an aerosol that goes with the sample and the gas support and the tube that is on the outside also contains gas from Argon to cool the tubes. A magnetic generator is responsible for producing a current that oscillates in the inductive coil (here refers to the name of the technique) this current is wrapped in the tubes, the inductive coil produces a magnetic field which in turn produces an oscillating where it establishes the current between ions and electrons of the gas, where they finally generate that energy to other gas atoms in the sample by means of various collisions that create a plasma with a fairly high temperature.
Going a little deeper into the subject we could know several methods of analysis such as: plasma of direct current, plasma induced by laser and plasma induced by microwaves.<7p>
Arc and electric spark, so that you can excite the atoms within the sample you need a spark or arc source as the name implies, this consists of a discharge of continuous electrical current between the electrodes of the instrument to be able to vaporize and subsequently excite atoms, these electrodes are usually made of metal or graphite, depending on the type of sample you want to analyze. This technique is widely used in the metal industry because of its great efficiency.
By means of this Arc or Spark method we can obtain very efficient emission spectra, it allows obtaining a qualitative and quantitative analysis of different metallic elements in different types of sample, be it soils, compounds, alloys, among others.
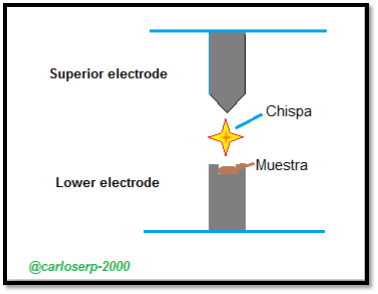
Outline of the process of the experimental method of Arc and Spark for the atomic emission of spectra
In previous years, the spark or arc conditions were not well controlled, the analysis of the elements of the sample was qualitative. However, sources of modern sparks with controlled discharges can be considered quantitative. Both qualitative and quantitative spark analysis are widely used for quality control of production in foundries and steel mills.
One of the important characteristics that the equipment must present is a good source of emission that can completely eliminate the sample of the analyzed material from the beginning to the end, that is, eliminate its original matrix in order to eliminate all possible interferences that may arise during the measurement phase. Important that the source of emission can perform an excellent atomization process, but minimum ionization of the sample to be able to perform a perfect sweep.
The source of emission must be effectively controlled so that it can then provide adequate energy and thus excite the atoms or molecules in the sample. It must have a range that includes organic and inorganic solvents, which can be attached to any type of material.
The environment surrounding the process must be totally free of impurities, suitable for the process. In general, a completely inert chemical medium is required that does not allow the formation of inadequate organisms that may cause damage to the analyzed sample, such as oxidizable material, carbides, among others.
And the most important thing without a doubt that you must have a source of emission is its operation that is easy, manageable and above all of low cost if necessary.
Now we can talk about some advantages that this experimental technique has and that one of the main advantages of the EES is without doubt its great efficiency, the errors are negligible, however, the preparation of the samples must be done with great care because the equipment is very sensitive to detect impurities that may cause damage to the measurements. At the same time, it can simultaneously analyze several elements in concentration of several orders of magnitude.
Some of the methods where this technique is addressed are such as:Agriculture and food, fertilizers, plant materials with the advantage that no specific treatment is needed for the preparation of the samples. Biology, for the analysis of different organisms. Clinic, for blood analysis, stool. The environment in order to detect any type of water pollution. They also include analysis of soil, sediments, animal and vegetable tissues.
And of course in physics and chemistry in the determination of concentrations of metals and non-metals.
And probably its only disadvantage is that the equipment to perform these measurements is quite expensive. Unlike EAA, fewer resources are needed to operate this type of experimental equipment.
If you want more information about the subject you can visit the following links:
Atomic emission spectroscopy Atomic absorption spectroscopy. Benchmark achievements of a decade Atomic Absorption Spectroscopy Emission spectrum Emission And Absorption Spectra Atomic Spectroscopy Spectroscopy: Interaction of light and matter Flame emission & atomic absorption spectroscopy Inductively Coupled Plasma Sources and Applications Inductively coupled plasma mass spectrometry IInductively Coupled Plasma Optical Emission Spectrometry. PDF. Xiandeng Hou College of Chemistry, Sichuan University, Chengdu,China Inductively coupled plasma mass spectrometryPublish through our official app and you will get an extra vote of 5% https://www.steemstem.io/
 Video credits @gtg
Video credits @gtg

This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and utopian-io!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 3 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 6 SBD worth and should receive 201 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePigHi @carloserp-2000!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Hi budy... Your post is very didactical. I´ll try to take profit of it... Greetings...
Congratulations @carloserp-2000! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOP