Spectroscopy Series VOL. 9: UV Spectroscopy
But before starting the content of this publication I suggest you visit my previous deliveries:
Ultraviolet-visible spectroscopy is one of the many spectroscopic techniques that are widely used today, particularly in the area of chemistry for the analytical study of various substances. UV is responsible for conducting studies through the interaction of electromagnetic radiation with matter, that is, half all the light that can not absorb a material through the wavelength used.
The primary objective of this technique of spectroscopy is to obtain a complete analysis of any chemical substance by means of a qualitative analysis that subsequently translates into a quantitative one in order to know the chemical concentration of the substance or material, that is, to know the quantity of the element chemical present in it.
It is possible to identify different functional groups of molecules to be able to analyze their content, strength and concentration of a substance, in turn in the quantitative analysis some ion components of transition metals and organic compounds especially those with a high degree of conjugation.
> As I have just mentioned, it is used mostly in chemistry and biochemistry laboratories to determine small amounts of certain substances, such as traces of metals in alloys or the concentration of a certain drug that can reach certain parts of the body.
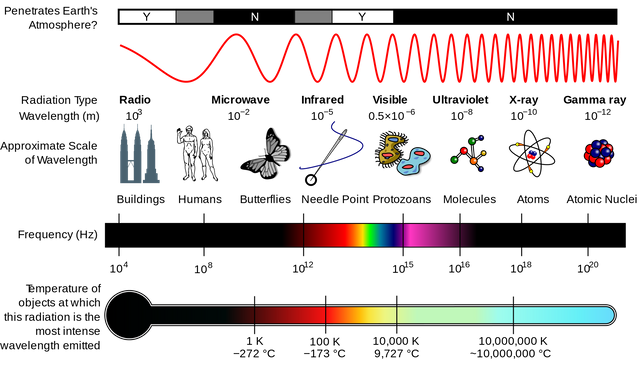
Electromagnetic spectrum Licensed CC BY-SA 3.0)
Everyone has ever heard about the electromagnetic spectrum in our lives, in particular it is part of our daily life and in every university career or even in our schools we have been told about it. We know perfectly well that the range of ultraviolet-visible radiation is between X-rays and infrared light. The ultraviolet radiation is located in the range between 190 and 400 nm, on the other hand, the visible radiation belongs to the range between 400 and 800 nm, it is important to note that in this region of the electromagnetic spectrum the wavelength carries different colors.
The absorption of radiation in the ultra-visible spectrum is associated with the electronic transitions of the energy levels of the atoms of the material, this means that the valence electrons can jump to another empty orbital that has a higher energy level, this if it is given the right energy to jump to that energy level. All this occurs by the absorption of a photon, where the transmitted energy must coincide between the ground state and the excited state.
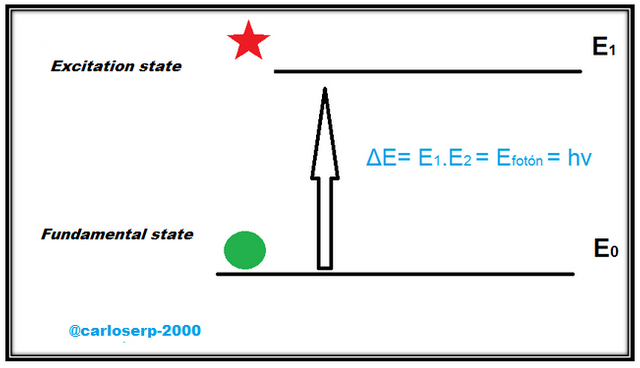
Absorption diagram of a photon, where the transmitted energy must coincide between the ground state and the excited state
We can say that the time of life of an atom excited by absorption is quite brief, we can say that the energy dissipates when the electron returns to its fundamental level, this is called the relaxation process. These relaxation processes can be non-radiant when the energy dissipates in the form of kinetic energy, in the form of heat or radiant relaxation processes when this energy tends to be released in the form of radiation.
When we proceed to perform the measurements by means of UV spectroscopy and then perform the spectral scan and graphically represent the radiation intensity of the substance as a function of its wavelength, we can obtain the characteristic spectrum of the substance that has been its fingerprint. or ID document, since it is unique to each substance. These spectra show maximums of characteristic energies at wavelengths with different intensities, all this is extremely important for the qualitative analysis, since, when studying the energy maximums of a characteristic spectrum of a specific substance, we can differentiate one from the other. And in turn also perform quantitative analysis to determine its concentration that has been greater wavelength at higher chemical concentration.

Relaxation process
The configuration for this technique is practically the same as other technologies mentioned in my previous deliveries, basically the same components are used to build the measurement system. In the following image we can observe a model of visible ultraviolet spectrometer.
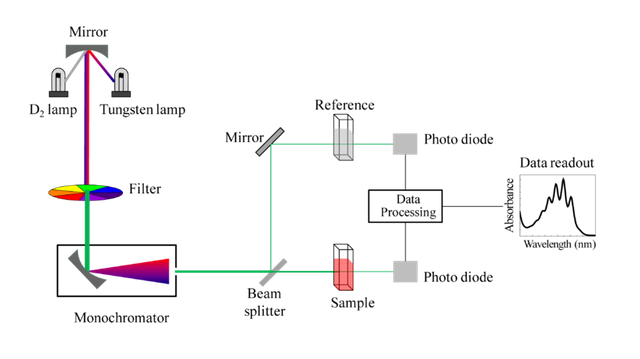
Schematic of UV- visible spectrophotometer Licensed CC BY-SA 3.0 of Wikipedia
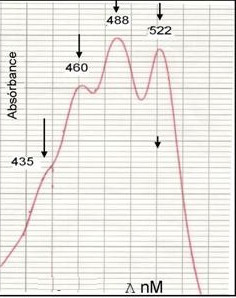
Example of Ultraviolet-Visible spectrum Licensed CC BY-SA 4.0
Now to understand in an easy way that this technique is treated for example, we have a substance, this must have color so that it can be visible, this means that the chemical absorbs frequencies and wavelengths of the visible range and allows to transmit other more , all this makes it possible for us to observe the color of a substance (it is the scientific explanation). A simple example where we can understand this nature is that we have available a substance of yellow color that belongs to the visible range and this absorbs a radiation of approximately 430 and 500 nm, in this range of the electromagnetic spectrum the wavelength is located in the color blue, that is, the substance absorbs the blue color and transmits other colors that complement and give rise to the yellow color.
The basic principle of UV spectroscopy is basically to measure the radiation absorbed by a substance in the UV range, ie the intensity of the color of a substance at a specific wavelength and then make a comparison with other known substances, it should be noted that they must contain the same absorbent species.
In order to obtain this relation between substances we need the well-known Beer-Lambert law, this law tells us that, for the same absorbing substance in a cell of constant thickness, the absorbance is directly proportional to the concentration of the substance, I will show later mathematically said relationship.
It is important to have knowledge that the color of a substance is related to a sample that is absorbent and this coloration may be specific to the substance or induced by us. It is very common to induce the formation of a compound with a lot of color and that can absorb in the visible range for example a colorful compound such as chlorine when reacting with another substance such as orthotuclidine, also the quantification of glucose in the blood and urine by the action of molybdate, the intensification of the color of the ion-copper to form an ammonia compound. For this you need to know certain important conditions such as: temperature, pH and time.
The method is most often used quantitatively to determine the concentrations of an absorbent species in solution, using Beer-Lambert's law:
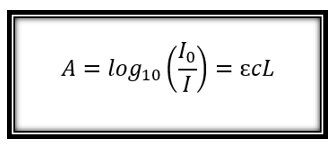
> where A is the absorbance measured (in UA absorbance units), the intensity of the incident light at a given wavelength, I0 is the intensity transmitted, L the length of the path through the sample and c the concentration of the absorbing species. For each species and wavelength, ε is a constant known as molar absorptivity or extinction coefficient.This constant is a fundamental molecular property in a given solvent, at a particular temperature and pressure, and has units of 1 μM x cm.
> Absorbance and extinction ε are sometimes defined in terms of the natural logarithm instead of the logarithm in base 10.
> The Beer-Lambert Law is useful for characterizing many compounds, but it is not a universal relation for the concentration and absorption of all substances. Sometimes, a second-order polynomial relationship between absorption and concentration is found for very large and complex molecules, such as organic dyes (xylenol orange or neutral red, for example).
It is important to highlight that Beer-Lambert's law presents a series of limitations in its study of absorption of chemical substances, for example this law can only describe in a correct and viable way the behavior of diluted solutions at a concentration lower than 10 milliMolar, this occurs because at greater concentration the distances between the molecules present in the substance decreases and begin to be affected electrostatically what happens to decrease the capacity of absorption.
Conclusion
We can say that UV spectroscopy is widely used in the field of chemistry and has an infinity of applications, it allows us to quantify substances, also to be able to achieve exact chemical concentrations through the interaction of electromagnetic radiation with matter. In analytical chemistry, the measure of the amount of light that a substance can absorb at a certain wavelength is quite interesting, since by means of Beer-Lambert's law this absorption can be related to its chemical concentration.
If you want more information about the subject you can visit the following links:
Ultraviolet–visible spectroscopy
Visible and Ultraviolet Spectroscopy
Spectroscopy: Interaction of light and matter
QUANTITATIVE CHEMICAL ANALYSIS
Beer lambert law the method is most often used in a

Video credits @gtg
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and utopian-io!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Hi @carloserp-2000!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV