Journey from adaptive immunity in bacteria(CRISPR) to saving Man's best friend(Dog).
Back in late 90s and early 2000s, when I learnt about Mendel and genetic diseases; for me genetic diseases were a one way street. If you inherited a gene with detrimental mutation, your fate was written in your DNA. Less, did I know that my view was going to change in this lifetime.

Picture taken and modified by @scienceblocks
By the time, I was in 12th grade(or junior college), I was keen on perusing genetic engineering. Well the restriction endonucleases, the enzymes that cut the DNA at specific regions, were a big thing back then. Today, it is just a tool I use in everyday work. Then came RNAi technology and gene therapy. The idea was to reduce the amount of expression of detrimental genes by targeting its product - the mRNA.
Well, apart from transgenic animals, which have genes inserted or deleted randomly in their genome, there did not exist a way to specifically edit the genes. But that was only until CRISPR-Cas9 technology came into the picture.
The Rise of the planet of CRISPR
You can think of CRISPR-Cas as an adaptive immune system of single cell organisms, such as bacteria. Like your immune system keeps a memory of pathogens you have been infected by, bacteria do it, as well. They keep sequences of pathogenic viruses(bacteriophages) and plasmids, in their DNA and use them to mount an immune response(Mojica et al., 2005). And it's quite interesting to know how it works.
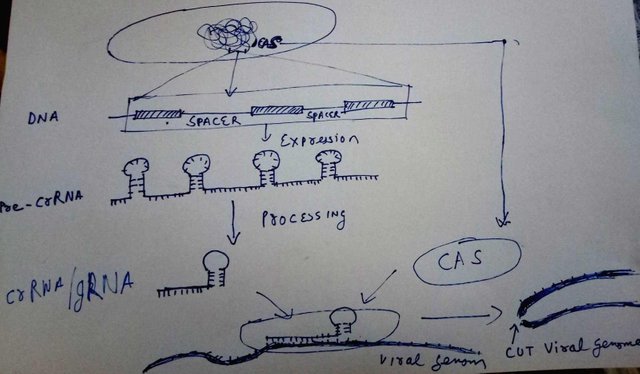
Doodled by @scienceblocks
The bacterial DNA contains a region called clustered regularly interspaced short palindromic repeats. So, it is a cluster of small DNA sequences repeated multiple times. However, between each repeat, there is a spacer. The spacer is a small fragment of genome, that this bacteria took from the virus, or a plamid that previously infected it.
Now, let's say that this bacteria was infected by 5 different kinds of viruses since the dawn of its time. It would have 5 spacers, flanked by CRISPR repeats. To use these spacers to kill the known virus genomes that enter the bacteria in future, it transcribes this CRISPR region, to make pre-CRISPR RNA or pre-crRNA. pre-crRNA is then processed and cut. Each cut contains one spacer and one repeat element. This assembly is what is called crRNA or guide RNA or gRNA.
The gRNA is recognised by an enzyme made in bacterial cells - called Cas. The gRNA homes inside the Cas enzyme, and helps Cas bind to specific regions on virus DNA, complementary to spacer region stored in bacterial genome. Once bound, it cuts the DNA of the virus killing its dreams to replicate within a bacteria.
So far, so good. And good for the damn bacteria. But what if I made gRNA specific to human genes, in the lab, and inserted it into human cells, along with the gene for CAS9. Well, then it should specifically cut the genes of my interest in them. And this is what is usually done when you hear about gene editing technology.
You pack a gene for CAS9 and gRNA in a vector(my preferred vector being lentivirus). Then you insert it into the cells of your choice. Inside the cells gRNA and Case together, cut and delete the genes that you want to remove. Alternatively, you can also use this strategy, to insert new genes. Because you are cutting the DNA and creating breaks in the genome, your cells will activate DNA repair pathway. The repair can happen by non-homologus pathway or via homologus recombination pathway. And, it is the later you want to cash upon. All I need to do now, is to add another gene of my interest, with flanking regions homologus to DNA I am cutting. And then? Then, I cross my fingers and hope that DNA repair pathway, will insert the gene of my interest in the DNA of human cells.(see Rath et al., 2015)
The journey of CRISPR technology
I mostly do gene editing in cultured cells. However, it has also been used to make genetically modified mice, and that too with precision of single nucleotide editing(Kim et al., 2017). It has been used to silence the genes associated with high cholesterol levels in mice(Thakore et al , 2018).
In march 2017, Yin et al.,), used CRISPR technology, to remove genome of HIV from the cells of live mice. If you don't know, then HIV is a retrovirus. Which means that post infection it inserts its genes into host's DNA. Hence, even if you could somehow target HIV virus particles, you may still be stuck with viral DNA in your cells. So the idea of Yin et al., was to remove the viral DNA from host cells. In order to achieve this, researchers packed CRISPR toolkit - the gRNA against HIV and the Cas9 gene in an Adenovirus. When they infected the mice with the armed Adenovirus, they were able to remove the HIV viral DNA from these mice.
Another proposed application of CRISPR deals with removal of latent viruses, such as herpes from human genome. About two weeks ago I published a blog, talking about how almost the entire human population, is infected by at least one strain of HERPES. Funny thing though is that these viruses can live and die with you, without ever causing any symptom. But, the incidence of these viruses waking up from sleep increases in outerspace(apart from old immunocompromised individuals). Which is not cool if you were an astronaut. And CRISPR provides a solution to this(see this article by Don Ward Hackett). Therefore, I guess it would be pretty good idea for NASA to consider investing in CRISPR research.
Anyway, as you might have noticed that most of these studies were done in mice, in laboratory settings. Before anything good can come of it for humans, its safety and efficacy has to be demonstrated in large animals, especially nonhuman-primates.
Saving the man's best friend.
While the studies of CRISPR's efficacy and safety continues in non-human primates, just 3 days ago, researchers used CRISPR to help dogs. They were able to repair the gene in causes muscular dystrophy - a disease in which the muscles waste away.
Similar disease is also found in humans. Duchenne muscular dystrophy(DMD) is caused by a loss of function mutation in dystrophin gene on the X chromosome(which is why it is more severe in males than females). Without proper medical care, boys with DMD hardly make beyond the teenage. The muscles in their body progressively degrade making them weaker everyday.
Dogs, like humans are also affected by mutation in dystrophin. The mutation in dogs stops the formation of a full length protein. So, in theory if you cut out this mutation and fix the gene.
Amoasil et al., made the gRNA to target this mutation. They packed it in adenovirus genome, along with gene for Cas9. They infected four affected dogs with this virus and measured the levels of dystrophin in their muscles, 8 weeks later.
Guess, what happened? Up to 90% recovery of dysyropin expression was found in these dogs. Now, the drawback of the study is that they just used 4 dogs and followed them for just two months. Nevertheless, it's a good case study in support of successful use of CRISPR technology in larger mammals.
I just hope that, soon this technology is also able to help over 100000 people suffering from DMD and millions of people affected by various genetic disorders, worldwide.
Listen to the CRISPR-CAS9 song
CRISPR-Cas9 ("Mr. Sandman" Parody) | A Capella Science
References
Signing off
@scienceblocks
Just a few years ago, maybe even now, they were teaching in undergrad courses that once a retrovirus is in, it stays. And now we can remove it using CRISPR. This alone speaks multitudes. Why governments don't throw everything they got at this research, boggles my mind.
The part about herpes waking up in space is simultaneously something I knew nothing about, and hilarious 😄
Really great overview of why CRISPR even exists and how it works. One thing that's always been an issue for gene therapy is delivery - do you have any plans to write up some of that?
Thankyou @effofex
I thought about writing it, when I was writing this post. I did slightly gave a hint of how adenovirus is used to deliver crispr toolkit into the cells of live animals. But, then I thought if anyone there would be interested in such details. Thanks for letting me know, I will do a post on it soon. :)
I'd very interested.
One slant to give it is 'we keep hearing about all these cool things, but never see them come to fruition... well, the devil is in the delivery.'
Hi @scienceblocks!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Love science posts.
I too have genetic disease IGA nethrophapy. After a lifetime of deterioration, I finally had a kidney transplant over a year ago from it. Doing well now.
upvoted your post
Joe
@joe.nobel
science fiction, fantasy, erotica
check out some posts, no obligation to upvote or follow
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
Hello there and thank you for your great work that adds value to the @steemstem community!
As we have stated many times, no user is allowed to get more than 3% of the total upvotes by the @steemstem account over a 14-day period. Following this general guideline, don't be discouraged if you see our voting percentages on your future posts dropping for a few days. It's not that we don't appreciate your work, we only make adjustments in order to be fair to all community members.
Thank you once again and have a great day!
I appreciate you letting me know about it. I was unaware of this. However, since I know now I will keep that in mind. Thankyou.
Reading your text reminds me the earliest post of @justtryme90. Very comprehensive, even for someone living far from genetics like me.
PS: I loved the white board ^^
Thankyou. Nice to know that I could convey the message regarding particles of life across. :)
I gotta go and do some digging on @justtryme90 posts and find the earliest posts and read them.
You may need to dig 2 years back in time. I am however sure you will have a lot of fun in doing so! ^^
Congratulations, your post had been chosen by curators of eSteem Encouragement program. Feel free to join and reach us via Discord channel if you have any questions or would like to contribute.
Also,
CREATING YOUR PROFILE IS EASY! JUST FOLLOW THE STEPS HERE ☜(ˆ▿ˆc)
You can trade your earned credstars for SBD!
Hi @scienceblocks!
Your post was upvoted by @steem-ua, new Steem dApp, using UserAuthority for algorithmic post curation! Your UA account score is currently 2.125 which ranks you at #21885 across all Steem accounts.
Your rank has risen 16 places in the last three days.
In our last Algorithmic Curation Round, consisting of 206 contributions, your post is ranked at #43.
Evaluation of your UA score:
Feel free to join our @steem-ua Discord server
Congratulations,
you just received a 16.66% upvote from @steemhq - Community Bot!
Wanna join and receive free upvotes yourself?
Vote for
steemhq.witnesson Steemit or directly on SteemConnect and join the Community Witness.This service was brought to you by SteemHQ.com