The Patterns of the Modern Periodic Table Part 2 (Let's Learn Series #20 - Chemistry)
Hello dear learners, today I'm gonna teach you some other things about the Periodic table. In my last post, I taught you how the periods and the Groups are divided. If you missed it, check it here.
Isotopes
As I told you before, the number of Protons and Electrons in the atoms of the same element doesn't change but the number of Neutrons change from atom to atom even in the same element. And as I told you before, the su of the number of Protons and the number of Neutrons is named as the Mass Number. So when the number of Neutrons varies from atom to atom, the mass number also varies from atom to atom in the same element.
The atoms with different mass numbers in the same element are known as Isotopes of that element.
Eg: Hydrogen has three isotopes. They are named as Protium (no neutrons), Deuterium (1 neutron) and Tritium (2 neutrons).
Patterns in the Periodic table
To learn about the patterns in the Periodic table, we have to consider the following properties of the elements.
- First ionisation energy
- Electronegativity
First ionisation energy
The negatively charged electrons are tightly attracted by the positively charged nucleus. So if we want to remove an electron from an atom, we have to provide the necessary energy which can overpass the energy of the nucleus. The least amount of energy that has to be supplied in order to remove an electron is known as the First ionisation energy. Then the atom becomes a positive ion. This can be done only when the elements are at the gaseous state.
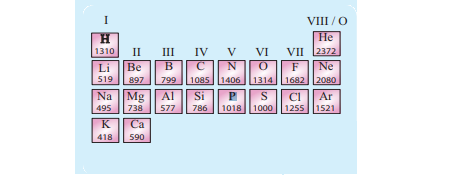
The following Periodic table shows the first ionisation energy of each of the first 20 elements.
If you look close, you will see that the Group i elements has the minimum first ionisation energy while the group viii elements have the maximum first ionisation energy. I will tell you why it is later.
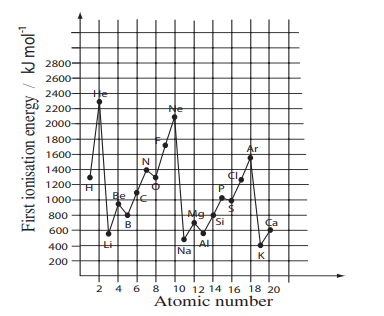
This graph shows the first ionisation energy of the first 20 elements.
Electronegativity
Electronegativity is the ability of an atom of a certain element to attract electrons of another atom of another element towards it. The element with the higher element becomes able of attracting electrons of the elements with less electronegativity.
This shows the electronegativity of the first 20 elements.
As you can see, the electronegativity of the Group i elements is less than the electronegativity of the group vii elements.
Now I'll tell you why the first ionisation energy of the group I elements is less than the first ionisation energy of the group viii elements and the electronegativity of group I elements are less than group vii elements.
Some of you might know, when an atom has all it's shells filled with electrons to their maximum level, they are known as Noble gases.
Ex: Helium has two electrons and a shell. So the only shell it has is filled to its maximum; the first shell can only bear 2 electrons and the first shell is filled to its maximum.
:Neon has 10 electrons and it has 2 shells and hey are filled to their maximum.
So every element likes to become noble. If we take Lithium, it has 3 electrons and the electronic configuration is 2, 1. So it has to either remove an electron or take 7 electrons to become a noble gas. Since it's easier to remove an electron, it has a low electronegativity value. So that the other elements can take an electron out of him easily.
If we take Sulphur, it has 16 electrons and 2, 8, 6 is its electronic configuration. So in order to become a noble gas, it has to either take 2 electrons or to remove 6 electrons. Since it is easier to take 2, it has a higher electronegativity value.
--------------------------------------------------------------------------------------------------------
Did you hear about the Teen community me, @mashiliyanage and @justaboutart are working on?
We created the #tos tag which stands for Teens On Steem
So that we can make Steemit a better place for teenagers.
So if you are a teenager, you can also use this tag.
Also, there's still time for the TOS contest. You don't have to worry thinking that you are not a teen, there's a contest for you guys too. You can check the contest here
***


#chamudiliyanage you are a good steemiter and I wish you good luck for your next days.
You can help me please I have no dollar so you can send me only 1 dollar please and i promise when i have some dollar i will back. Because I am new member but i ready 31 post but no deblope. So Please dont mind You can do it??
Ok no problem @sakibhossain, but I believe that you are not cheating me. I don't mind giving you a dollar. Btw, can you contact me via Discord, Twitter or Steemitchat?
thank you so much friend and whats name on your twitter ???
chamudiliyanage
This lesson remembers my school days. Keep continuing @chamudiliyanage it will help your academics also.
Thank you @shirosh. Of course, this is like a revision for me so I just don't have to study for months :D
This is such an amazing post! I can tell you put time and effort into this, and well...that's also amazing! It's definitely a reminder; I haven't actually study about atoms in like 2-3 years, so its good to get the recap. And well to be honest about another thing, I had no clue there was a such thing as "first ionization energy," so thanks for that!
Thanks but do you really want to remind these things again? Reminding the things that you no more have to study about is just a big headache for me. Anyway, glad to hear that you learnt something from my post :D
Well of course! The world is fascinating itself, but when you start talking about it's building blocks, it normally almost intrigues me...unless i don't get it haha. Anyways, learning about our and our universe's building blocks is pretty cool, so keep this up! I think i'm gonna be a regular to this series :D
Thank you Justin.
https://steemit.com/steemstem/@steemstem/guidelines-on-copyright-standards-in-steemstem