The Patterns of the Modern Periodic Table (Let's Learn Series #19 - Chemistry)
Hello dear learners, I am back with my Let's learn series and today, I'm gonna teach you a Chemistry lesson regarding one of the main things, The Periodic Table.
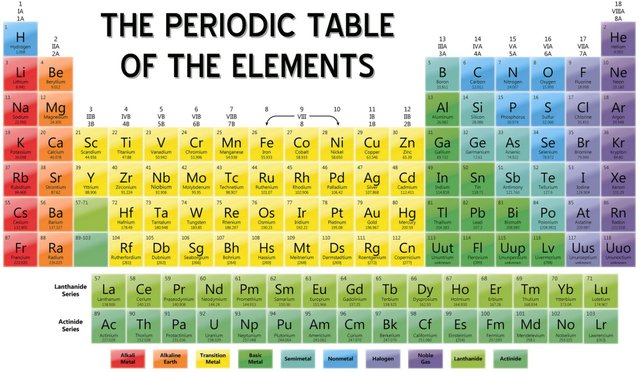
So, as most of you know, it is a table of 118 elements arranged in a particular manner. The modern periodic table is introduced by the great scientist, Dmitri Mendeleev. This is one of the things that you must know if you want to learn Chemistry.
This is the periodic table
Today, I'm gonna teach you about the first 20 elements in the periodic table.
Before I move to the periodic table, there is some other things I should teach you.
The planetary model of the atom
Earlier, it was said that the atom is the smallest unit of matter. But now, it is revealed that the atom contains Protones, Electrones and Neutrones. The Protons has a positive charge, electrons are negatively charged and the neutrons have no charge. When this is said, scientists doubted how these Protones, neutrones and the electrons are placed in the atoms. In 1911, the great scientist, Ernest Rutherford introduced the Planetary model of the atom.
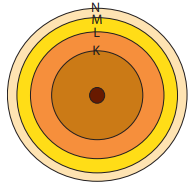
According to the Planetary model, the neutrons and Protons are situated in the nucleus and the Electrons move around the nucleus in definite paths. (The atom is neutral) As Niels Bohr said, the pths, shells or energy levels are named as K, L, M, N...
There is a definite number of electrons that can be situated in each energy level.
- K - 2
- L - 8
- M - 8
- N - 18
Atomic number
The atomic number of an atom is the number of Protons in an atom.
P.S. The number of Protons and Electrons are same in the atoms of the same element but the no. of neutrons are different.
eg: in a nitrogen atom, there are 7 protons and 7 electrons.
in an Oxygen atom, there are 8 protons and 8 electrons.
So the atomic number of Nitrogen is 7. The atomic number of Oxygen is 8.
The symbol for the atomic number is Z
Mass number
The mass number of an atom is the sum of protons and neutrons.
The symbol for the mass number is A
The atomic number of Sodium is 11. So the no. of Protons in Sodium is 11. If it has 12 neutrons, the mass number is,
11+12=23
This is the standard way of writing the atomic number and the mass number of an element.

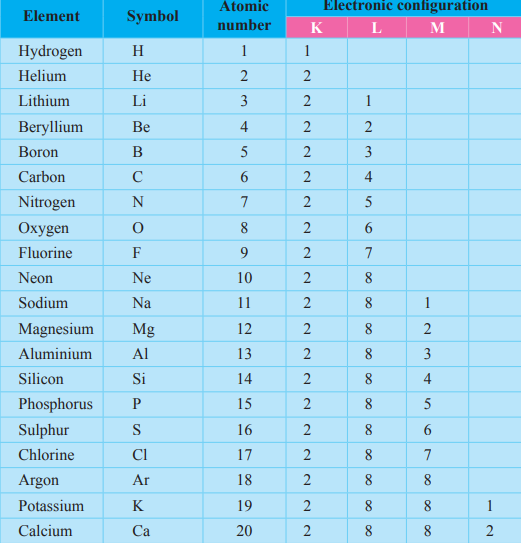
Electronic configuration
Representing how the electrons are filled in the respective energy levels is called Electronic configuration.
eg: Sodium has 11 electrons Na - 2, 8, 1
Magnesium has 12 electrons Mg- 2, 8, 2
Carbon has 6 electrons C - 2, 4
Now let's again look at the periodic table.
Here, the vertical rows are named as Groups and the horizontal rows are named as Periods.
So, there are viii groups and 4 periods. (groups are numbered in roman numbers while the periods are numbered in arabic numbers.)
Dividing elements into groups
In this, we need to consider the electronic configuration of the elements.
The electrons at the outermost energy level is known as Valence electrons. The elements are divided into groups considering the valence electrons of the elements.
Let's try it with examples.
- electronic configuration of O - 2, 6
- Valence electrons - 6
- Group number - vi
- Electronic configuration of Na - 2, 8, 1
- Valence electrons - 1
- Group number - i
- Electronic configuration of N - 2, 5
- Valence electrons - 5
- Group number - v
Like that, the no. of valence electrons is the Group number of that element.
Dividing elements into periods
When dividing into periods, the number of energy levels are considered.
eg:
- Electronic configuration of O - 2,6
- Number of shells - 2
- Period number - 2
- Electronic configuration of Mg - 2, 8, 2
- Number of shells - 3
- Period number - 3
--------------------------------------------------------------------------------------------------------
Did you hear about the Teen community me, @mashiliyanage and @justaboutart are working on?
We created the #tos tag which stands for Teens On Steem
So that we can make Steemit a better place for teenagers.
So if you are a teenager, you can also use this tag.
Also, there's still time for the TOS contest. You don't have to worry thinking that you are not a teen, there's a contest for you guys too. You can check the contest here
***
I bet Sodium and Chlorine like to hang around with each other. Sodium has one electron in its out shell and Chlorine has 7 but wants 8. So if they get together, then Sodium can lend its one electron to Chlorine and they will both be very happy. I wonder what happens when they get together? :)
Very good Chemistry lesson!
Haha, yeah they'll be very happy to become a noble gas. You are very educative. I hope to post about the First ionization energy and electronegativity in a future post.
Thanks a lot ^^
They might turn into salt if they get together! Maybe you will do a Chemistry experiment and find out. :)
Yeah, Sodium Chloride means Salt right? Yeah, I'll try it out but I think I'll need more equipments and also I'm a bit scared of trying it cuz a fire is generated. Anyway, I'll try it in the future and post a video on it :D
Reading your article about periodic table system feels like I just turned back my memory on to my junior school years.
I first learned those things at this stage before. Thanks for sharing little girl @chamudiliyanage ❤️ Hi @kenny-crane, I think we've met in many posts. Pardon me 🙏🏻
You are welcome @fitrianizar
Love the science here and it is good to know the elements. I think humans are made up of water and carbon and potassium and maybe one or two more things, basically, mostly. To be healthy, we may want to make sure we have those basic elements in our bodies first, at least. Thanks for sharing, hehe. I'm Oatmeal Joey Arnold. You can call me Joey.
Hi Joey, yes, we are made up of Oxygen, Nitrogen, Hydrogen, Carbon, Calcium, Potassium, Phosphorous and so many elements. Thanks for stopping by ^^
Right, agreed, those are the top seven elements inside people. I love the number seven. Lucky number seven. I drink water. I try to get calcium. Bananas have potassium.