Fascinating genetics #Cloning vectors, The plasmid.
Fascinating genetics #Cloning vectors
The plasmid
Greetings friends, Time has come to introduce you to the next fascinating and all so important tool for any one working with genetic engineering. It is the Cloning vector, and most importantly, The Plasmid.

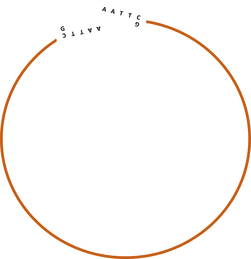
Plasmid, Consisting of doubblestranded DNA in a closed circle conformation.
Image Plasmid
A cloning vector is a small wonder of nature itself and is defined as a carrier of genetic information. One type comes fully functional directly from bacteria such as e.coli, and is called plasmids. Plasmids are used by bacteria to exchange genetic properties such as immunity to certain antibiotics. There are other kinds of cloning vectors used to insert DNA into organisms, such as bacteriophages which mimics viruses that infects bacteria and cosmids, which is a fusion one could say.
In this post we will look closer at how we commonly use plasmids in genetic engineering today as well as take a look at two different restriction enzymes, which are used to cut the circular plasmid open.
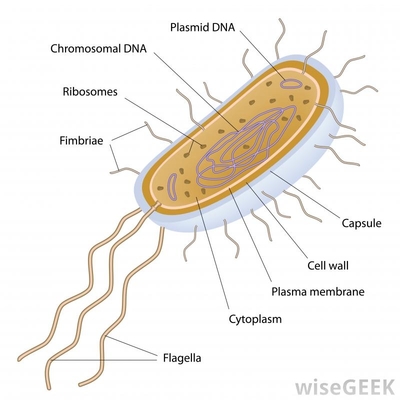
Image Bacteria
When working with proteins it is very important to be able to grow the protein in large quantities in bacteria, since it is very hard to perform any measurements on to small volumes. The same idea goes for setting up protein growing operations of different scales. The problem tho, is that bacteria does not naturally express any protein of interest so we must fix this by cloning.
To do this, we simply use a cloning vector, most commonly a bacterial plasmid, to insert our desired gene, which carries the DNA code for our needed protein, in to super potent E.coli bacteria. The super potent E.coli are easier for the plasmids to enter via the membrane, which gives higher success rates of transformation, when the plasmid penetrates the membrane.
The gene can be copied by PCR, a fast procedure, which i wrote about here to retreive sufficent amounts DNA for inserting in into the plasmid via liagtion as we call it when the gene is inserted into a plasmid.
The cloning vectors we use are most commonly on of two kinds, either a plasmid or a lambda phage. The plasmid is the natural mechanism by with bacteria exchange genetic material with each other. They are naturally capable of penetrating the membranes of E.coli, so we simply mix them together after finishing the plasmid design by inserting the gene.
A typical cloning plasmid consists of circular dsDNA, short for double stranded DNA with a known sequence. Knowing the sequence is very important in order to choose the right restriction enzymes, they must have a specific matching sequence to bind to.
In nature, there are enzymes in the bacteria, called restriction enzymes, and they are dedicated to cut in plasmids at certain base pair sequences, e.g ATTA might be a target.
We must therefore chose a plasmid with a known restriction map, meaning fully sequenced so we can know exactly where the restriction enzymes will cut and if it will cut, along with how the open ends will be structured. There are 100´s of restriction enzymes and the binding spot for the ones capable of cutting a vector is illustrated in its restriction map, like for the plasmid PET322 below.
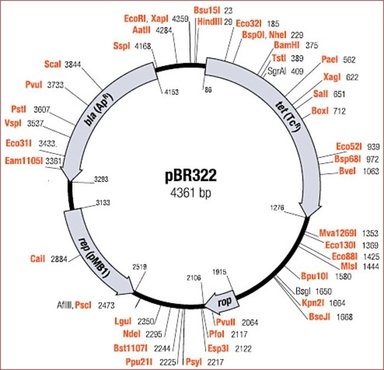
Order Pet322 and Image
The different names like EcoRI or BamHI are different restriction enzymes and the names are positioned at the specific cutting sequence for each enzyme. The enzymes leaves different ends on the plasmid when they cut the circular structure open. To obtain good results we use two enzymes and cut out the part in between their respective binding spots. The enzymes should be chosen to minimize risk of self ligation, meaning that the open plasmids close automatically again, and hinders the insertion of the DNA fragment.
Different enzymes leave different endings so we can easy calculate the risk of self ligation here. If the odds are to high for some reason, there are reversible reactions that can be carried out to protect the ends and prevent self ligation.
Except for the binding spots and what kind of ends the enzymes leave, there are some other properties that are important when working with e.g growing proteins. There must be a certain promotor, a region in the DNA of the plasmids, that induce transcription, meaning that the DNA is actually converted into a protein. Otherwise we would get little over no yield of protein, which is useless for most projects to say the least.
Inorder to quickly know if a certain plasmid was successfully inserted and expressed, the plasmid comes with resistance against a certain antibiotic, mostly commonly ampicillin. This gives the possibility to grow the bacteria in ampicillin medium, making sure that only the bacteria carrying the plasmid can live here, and that no unnecessary bacteria take up space.
To also know if the insertion of the desired gene into the plasmid was successful we can simply run a new PCR cloning procedure, but this time using our bacteria as template. We simply lyse the bacteria in a PCR tube and run a few cycles with the same primers as we used for cloning. Any amplified DNA will confirm the ligation process, since no other DNA sequences can be amplified with these primers.
BamHI & EcoRI, Restriction enzymes
As a specialist in proteomics i feel obligated to present the proteins a bit closer, so here is the functions of the very common restriction enzymes BamHI & EcoRI.
As mentioned the enzymes binds to specific sequences and BamHI are specific to the sequence 5'-GGATCC-3'. It will cut between they two G´s in the 5´- 3´direction, leaving an underhang. The top sequence will only gave the G left, the bottom will have CCTAG and this extra tail is called underhang and prevents the automatic ligation, unless the the other enzyme leaves a complementary overhang.
3'C C T A G ↑G 5'
If we chose EcoRI as the second enzyme, we will cut out the piece in between their binding spots. EcoRI will also leave an under hang, and double underhangs will not self ligate much, so these are good choices when working with the vector PET322
3'C T T A A ↑G 5'
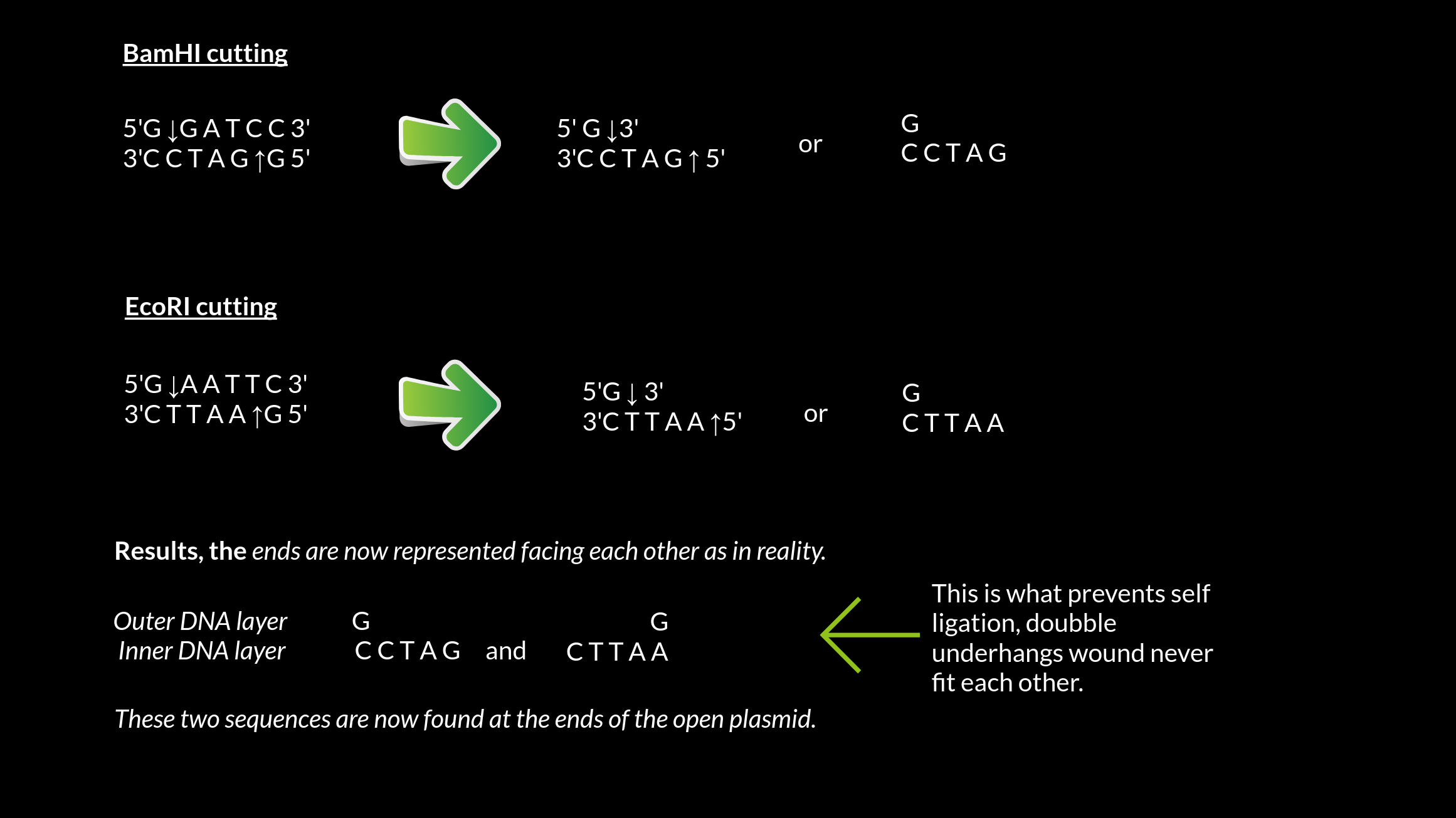.png)
These two tails will not be able to bind, and we can move on to inserting our DNA without treating the ends to protect against self ligation.
The DNA that we want to insert must be designed to fit this spot exactly, and we simply add the complementary sequence of the under hangs to our gene when preforming PRC.
It is done by designing the primers, the fragments that induce replication, with the extra sequence needed, added on.
Schematic representation of plasmid cutting and ligation:
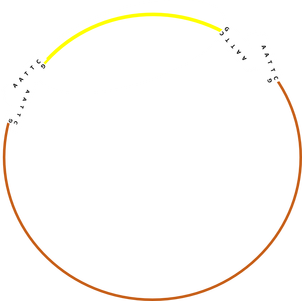
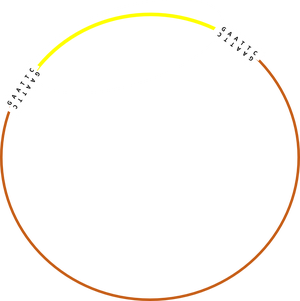
Shopping list
Have fun cloning and growing some proteins
My previous episodes on Genetics & Proteins:
Life science reviews:
Epigenetic effects of arsenic exposure
Fascinating genetics:
The Polymerase chain reaction
Telomere & telomerase
Retrotransposons
Fascinating proteins:
Aquaporines
ATPsyntase
The Prion protein
Amyloid precursor protein, APP & Alzhemier´s
Short news on:
Dangers of glyphosate
Cancer progression from arsenic exposure
Kudos on this remarkable post on genetics. This is such an interesting topic and so much more advancement ahead. I always tease my husband by saying that I would love to clone my kitty because I love his personality so much. I hope that we will be able to utilize scientific knowledge to prevent and cure chronic conditions.
Thanks for checking it out! Haha i would love me some cloning as well, but I fear that personality might be hard to preserve a 100% with memories and what not :)
I think fighting hereditary diseases with genetics is a good start to eliminate the worst kinds of diseases indeed !
Peace
I was just going to do a study on that same subject. What a coincidence.Its taken me this long to find that video, interruptions fall like rain. I'll send more links if you found this one helpfull
I ll check it out in a sec thx!
Very interesting! Have I resteemed!
Thanks :)
Your post was resteemed👍
I love science, if you want to read why i think steem can change the world please follow me and read my blog
thanks for having a look :) I ll sure check it out!
Peace
Good post, I am a photographer, it passes for my blog and sees my content, I hope that it should be of your taste, you have my vote :D greetings
Thanks :) i ll check in!
Bloody good read as always!
Yeah plasmids & recombinant DNA tech always interests me :)
Peace
Hello! Pretty sad that CRISPR CAS9 didn't win a NOBEL prize...!
I think there is still too much controversy going around about who discovered CRISPR/Cas9 versus who figured out its best use. Additionally, the Nobel prize isn't awarded posthumously, so I think priority was given to some people who had amazing contributions to the field but are near the end of their life.. The CRISPR groups are all young :)
Its about time :)
upvote and resteem