The Aβ protein- Alzheimer´s disease
Alzheimer´s disease & The Aβ protein
This is the first post on a specific amyloid protein and an associated disease, Alzheimer's disease caused by APP, Amyoild precursor protein, and its cleaving process in the cellular membrane. High concentration APP can be found around synapses at our neurons making the brain most likely to be affected. This little series of posts aims to both raise awareness on amyloidosis and what causes it. Since it both have horrific global consequences, and that some serious funding is needed here I think it´s worth spending some time on.
The other aspect is to introduce different proteins and the effects they can have, to change how many view proteins. I think that many see proteins as just nutrition and also might think that all proteins is similar and nonfunctional. In truth, the human body contain over 17000 different proteins, each capable of performing different, specific and complex actions or reactions that or body needs. There are many known disease, called prion diseases, caused by eating animal or human proteins, such as: Kufu and Mad Cow disease, which the next post on the topic will dive into in more detail, but for now, Alzheimer's Disease.
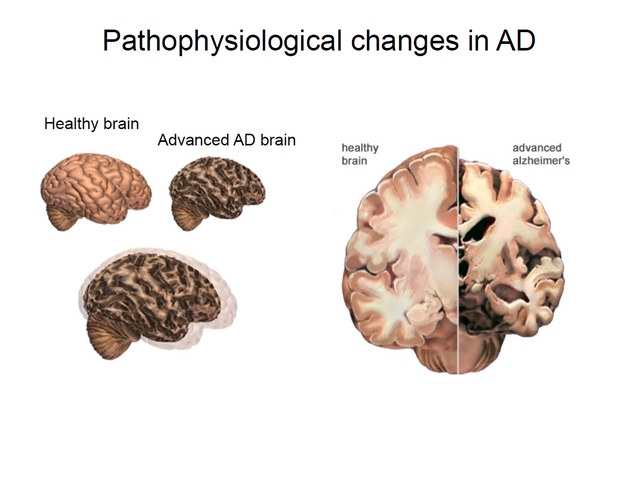
Alzheimer's disease as a cause of death has surged almost 100% since year 2000. Seen in the picture above is the evolution of some common death causes between 2000 and 2014. While the risk of death as a result of cancers,heart disease & HIV has decreased, the deaths caused by Alzheimer´s disease has doubled. It's not only a cause of death but also of social suffering, when e.g memory loss(dementia) and behavior changes occur. The costs of nursing Alzheimer patients are already around 200 billion in the USA alone, affecting over 5 million individuals.
- Every 60 seconds someone develops Alzheimer's disease!
- Alzheimer's disease is the sixth or seventh leading death cause.
- Some natural medicines show protective effects.
Alzheimer's disease was theorized around 1901 by Louise Alzheimer who found tangles and fibers when performing an autopsy on a woman suffering severe mental disorders. It is still not cured!
AD, or Alzheimer's disease is caused by mutations or age related effects of a protein called APP or amyloid precursor protein, it is present in the membrane of all our nerve cells. This protein is cleaved in the membrane by a protein called β-Secretase or α-Secretase which is followed by another cleaving by γ-Secretase.
If β-secretase cuts the protein before γ-secretase it will result in a small fragment, a peptide, 40 or 42 amino acids long, called Aβ40 or 42. The length is dependent of where γ-secretase cuts, look at the picture above and you can see 2 different cleaving sites for γ-secretase at positions 40 and 42.
If α-secretase cuts before γ, till will instead generate a peptide called called p3, which is non amyloidogenic.
The two different outcomes can be seen here , in the picture above, P3 fragments to he left, in the non amyloidogenic pathway, and the Aβ peptides to the right.
So what makes Aβ so dangerous compared to p3 fragment, which is almost identical ?
Well since amyloidosis is the result of protein aggregation, the problem with Aβ is that it is prone to aggregate and turn into amyloidogenic peptides and seeds.
There are also many known mutations making the protein fragment, Aβ, more likely to aggregate. There are interestingly enough also known protective mutations, which would make this progress less extensive and perhaps not dangerous at all.
Many mutations in the first part of the 40-42 amino acid long sequence has been found and also linked to increased Aβ levels, or produce to much Aβ42, causing a dangerous raito.
This has been methodically studied and points to the importance of hydrophobic effects at the middle region of amino acids when it comes to stabilization of fibril formations. The N-terminal region, or first region, seem to to important for β-secretase binding since mutations here increase the levers of Aβ.
The Swedish mutation, E693G and the English mutation on position 5 respectively 10 in the Aβ fragment gives rise to distinct pathogenic progressions including faster Alzheimer progression.
The middle region with mutations as the Dutch and arctic is known to be protective, and results in lower levels of Aβ, but still leads to AD.
As time progresses the ratio of β to α-secretase seems to be altered, why is not exactly known, but genetic and environmental factors seems to both be of importance in old age progression of Alzheimer's.
The aggregation and fibril / plaque growth is not the only problem, the Aβ40/42 fragments is neuro toxic by themselves and the cells must get rid of them in order to not suffer apoptosis, cellular death.
Credit for this [Figure] @S. M Strittmatter, Director, Yale Alzheimer's Disease Research Center (http://medicine.yale.edu/neurology/people/stephen_strittmatter.profile)
In the picture above we can see the non aggregated AB fragments hurts mitochondria by inducing oxidative stress. They bind to and suppresses g-protein signaling. The smaller aggregates and polymers, meaning more than one Aβ bound together, can also get stuck in different pores, thereby killing the cell. If the polymers exit the cell it can bind to blood cells in the brain causing hemorrhaging strokes. The aggregates can also create pores making calcium flow into the cell, killing it. They also interact with metals which causes wide spread problems when Cu and Fe levels drop.
Mutations doesn't only make the AB peptides fold bad or aggregate but might also have effect on the possibility for other proteins, or enzymes as we call them, to degrade AB.
NEP and IDE, or rather the proteases Neprilysin and Insulin-degrading enzyme both have the capacity to cleave and thereby degrade Aβ peptides.
When the undigested Aβ polymeres exit the cell the aggregation in the brain starts due to building of amyloid fibrils, made of repeating parallel β-sheet structure as seen below. The Aβ-fragments should fold into motif with some α-helice content , so the beta strand formation might be an important clue.
These fibrils is made of misfolded Aβ peptides that form polymers and then grow along a fibril axis. The fibrils grow into mature plaques in the brain and can also cause the binding of other proteins or protein fragments to the plaque, leading to different plaque composition in different humans.
There is much more that could be said about the fibrils and different step of formation but i think this will do here :)
The result of alzheimer's progression is death of neurons, and plaque / tangle formation killing the brain from the inside out.
Everyone might be affected at old age, but the mutations make the progress start much earlier so science is in dire need of subjects to study, check your DNA, even as a healthy individual you can contribute as control specimen.
How do we cure this ? Since we know of protective mutations, gene therapy would not be far fetched. We also know which enzymes is involved making it possible to target these for silencing with mRNAi, rendering the proportion more in favor of α-secretase.
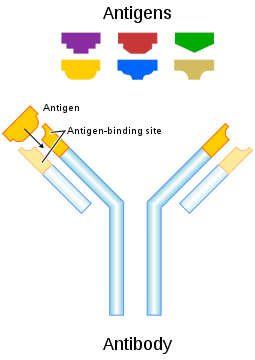
Targeting Alzheimer's on a non-genetic plane is harder and currently antibodies is in focus.
The antibodies is designed for binding to the Alzheimer's plaques in the brain. The antibodies they works as a tag, which will make macrophages devour the plaque. The problem is specificity, since the plaques have different compositions, it is hard to design an antibody that will target nothing but these plaques.
There are nonetheless many phase 2 and 3 trials being conducted on antibody treatment of AD.
Figure
To increase specificity i would design an antibody conjugated protease, targeting Aβ42 primarily, since most known mutations result in a bad ratio of Aβ42 to Aβ40 resulting in dimerisation/aggregation. A design for Aβ in general would also be good since Aβ40 or 42 by themselves can be neuro toxic at to high concentrations. The protease, preferably a modified Neprilysin specific for Aβ that folds upon binding to the designated part. The folding would activate the protease and leave it inactive when its not bound to the right plaque or peptide, solving specificity problems.
Some other therapeutic methods that has / is being tried can be seen here, i made a slide since i wont go in to any more detail.
There are two different natural products present along with vitamin E. I am personally fascinated by the effects of Curcumin, so try using some turmeric in your food, or if you don´t like it in more than one or two dishes, do like me and drink tea containing it on a daily basis.

Curcumin has been both proposed and partially proven to have positive effects on many other diseases as well so don´t miss out!
Heck, why not throw in a ginko berry and some vitamine E when you are at it, I hope it helps! :)

Wow, i wouldn´t wanna know if i had one of these mutations, who would ?
Great post thanks!
No, i agree that this information might be a bit heavy on most minds, but i will also enable you to search help in time. You could just eat tumeric and hope :)
Thanks for checking the post out!
You got a 4.24% upvote from @votebuster courtesy of @clausewitz!
This post has received a 0.03 % upvote from @drotto thanks to: @banjo.
Ty
0.35% @pushup from @clausewitz
@OriginalWorks
The @OriginalWorks bot has determined this post by @clausewitz to be original material and upvoted it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Clausewitz from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP. Be sure to leave at least 50SP undelegated on your account.
Sneaky Ninja Attack! You have been defended with a 0.68% vote... I was summoned by @clausewitz! I have done their bidding and now I will vanish...Whoosh
Have you ever walked along a shoreline, only to have your footprints washed away? That's what Alzheimer's is like. The waves erase the marks we leave behind, all the sand castles. Some days are better than others.
The art and science of asking questions is the source of all knowledge.
The notion of superhumans is using bioengineering and artificial intelligence to upgrade human abilities. If they use the power to change themselves, to change their own minds, their own desires, then we have no idea what they will want to do.