Overview on Thermodynamic Processes with Review Problem (English Units): Isentropic Process
Hi folks!
This is the fourth blog that I created which tackles about the five thermodynamic processes wherein I discuss what the process all about is and then the mathematical equations that governed the thermodynamic process and followed by a review problem. And here are the five thermodynamic processes:
- Isometric Process (Constant Volume Process)
- Isobaric Process (Constant Pressure Process)
- Isothermal Process (Constant Temperature Process)
- Isentropic Process (Constant Entropy Process)
- Polytropic Process
I am done creating blog about the first three thermodynamic processes (isometric, isobaric and isothermal), so today allow me to share my thoughts and knowledge about isentropic process.
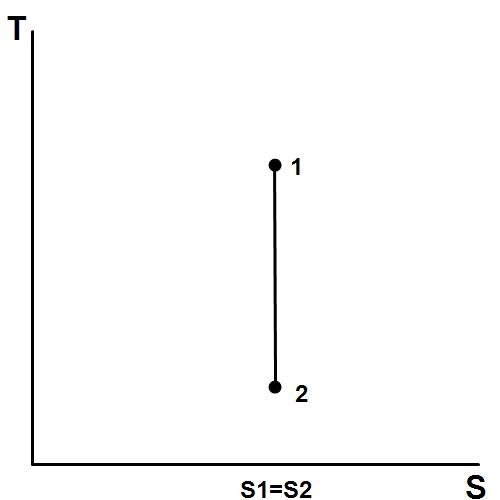
WHAT IS AN ISENTROPIC PROCESS?
An isentropic process is a thermodynamic processes wherein there is no heat being produced during change in state. This process is sometimes known as a reversible adiabatic process, adiabatic in the sense that it does no heat. This thermodynamic process is a reversible one. And lastly, this thermodynamic process follows the general assumption that:
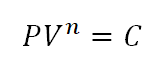
n is substituted with k which is the adiabatic index of a substance like ideal gases, the equation above justifies that it is a reversible adiabatic process or isentropic process.For mechanical engineers and mechanical engineering students, isentropic processes is often exhibited in almost all of the known thermodynamic cycles starting from the Carnot Cycle, Otto Cycle, Diesel Cycle, Brayton Cycle, Rankine Cycle and so on and so forth.
Moving forward, let us now proceed to the mathematical equations that support this thermodynamic process. And these mathematical equations are as follows:
- Pressure (P), Volume (V) and Temperature (T) Relations
Additionally, for the Temperature (T) and Pressure (P), it is derived from the first equation wherein the volumes (V) are expressed in terms of pressures (P).
- Nonflow Work (WN)
The formula for the nonflow work is given by the following equations (the second is derived from the universal gas constant formula):
- Change in Internal Energy (delta U)
As always the change in internal energy is expressed as a function of specific heat capacity at constant volume (cv) and the change in temperature (delta T).
- Heat Transferred (Q)
As mentioned that in an isentropic process, there is no heat during change in state.
- Change in Enthalpy (delta H)
As always the change in enthalpy is expressed as a function of specific heat capacity at constant pressure (cp) and the change in temperature (delta T).
- Change in Entropy (delta S)
Since this ia a constant entropy process, thus change in entropy is zero.
- Steady Flow Work (WS)
For the computation on the steady flow work of an isentropic process, the only thing to do is to have an energy balance based on the law of conservation wherein the energy entering is always equal to the energy leaving the system. And steady flow work is obtained as shown in the photo below:
And let us now proceed to the last and most interesting part in this overview on isentropic process and that is the presentation of a review problem. And the review problem goes by the statement found below:
One pound of an ideal gas undergoes an isentropic process from 95.3 psig and a volume of 0.6 ft3 to a final volume of 3.6 ft3. If cp = 0.124 BTU per lbm per unit degree Rankine and cv = 0.093 BTU per lbm per unit degree Rankine, what are the:
Temperature at the final state, (T2)Pressure at the final state, (P2)Change in enthalpy, (delta H)Nonflow work, (WN)
So let us now start computing these parameters that are being asked by the review problem.
Temperature at the final state, (T2)
In solving for this portion of the review problem, this can be easily solved by using the Temperature (T) and Volume (V) relations under isentropic process. But since we are only provided with gage pressure (P1), volume (V1) and mass (m), we need to obtain the initial temperature (T1) of the ideal gas by using the universal gas constant. From the universal gas constant formula,PV = mRT, we found out that we weren’t provided with gas constant (R) of the ideal gas but thankfully we are provided with both the specific heat capacity of the ideal gas at constant pressure (cv) and at constant volume (cp), respectively. So with that we need to obtain first the gas constant by using the relation of cp, cv and R. And computation goes like this:
And the we have computed that R is equal to 24.118 foot pound force per unit pound mass per unit degree Rankine.
By now we can now get the temperature during the initial state (T1) of the ideal gas by using the gas constant formula and the computation goes like this:
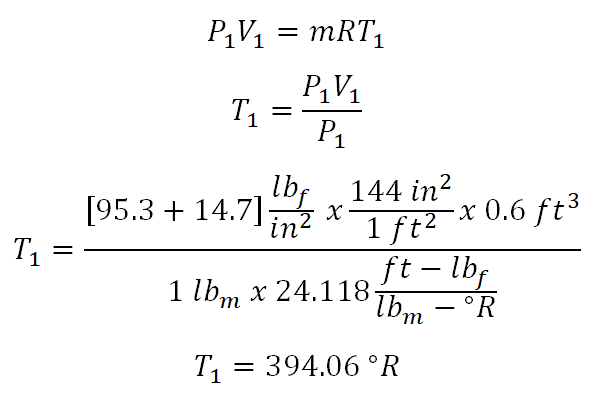
394.06 degree Rankine. Take note also that I added 14.7 to the initial pressure which is 95.3 psig, the very reason for that is the provided pressure is gage pressure and when using the universal gas constant formula both the pressure (P) and temperature (T) must be expressed in absolute, say absolute pressure in psia and absolute temperature in degree Rankine.Before we can solve for the temperature at the final state, we need to obtain first the adiabatic index (k) which is expressed as the ratio between the specific heat capacity at constant pressure (cp) and the specific heat capacity at constant volume (cv) of the ideal gas. And the computation goes like this:
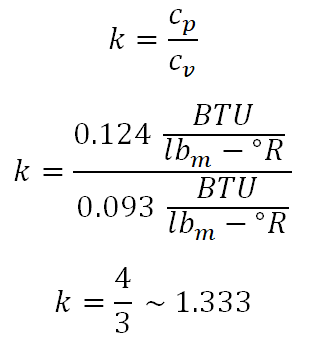
1.333.Finally, we now have all the necessary parameters in obtaining the temperature at the final state and the computation goes like this:
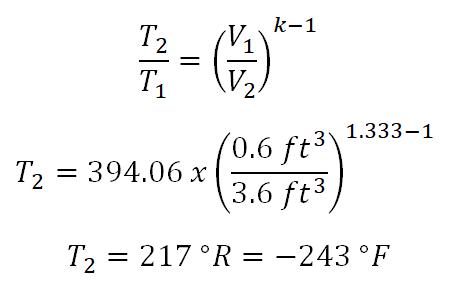
217 degree Rankine or -243 degree Fahrenheit.Pressure at the final state, (P2)
In the computation of this portion of the review problem, we are just going to use the relation ofpressure(P) andvolume(V) at isentropic process. And the computation goes like this:
And we have an absolute pressure of 10.10 psiaduring the final state of the ideal gas.
Change in enthalpy, (delta H)
The change in enthalpy (delta H) being experienced by the ideal gas is easily obtained thru using the formula for the change in enthalpy and directly substituting the needed parameters. And the computation goes like this:
Thus the change in enthalpy being exhibited by the ideal gas during change of state is equal to -21.96 BTUand that indicates a decrease in enthalpy.Nonflow work, (WN)
As for the solution in obtaining the nonflow work (WN), I selected first the one wherein it utilizes the following parameters: Pressure (P), Volume (V) and adiabatic index (k). And the computation goes like this:
And the nonflow work being done by the ideal gas during change in state is equal to 16.47 BTU.
Additionally, I made a computation also for the nonflow work (WN) wherein it uses the following parameters: mass (m), gas constant (R), temperature (T) and lastly, the adiabatic index (k). And the computation goes like this:
And there is a slight difference between the two computed value of the nonflow work (WN) done by the ideal gas during change in state and that is pretty negligible which is 0.01.
I guess that would be all regarding this blog post of mine which tackles about the isentropic process of substances like ideal gases and steam.
Thank you for spending your precious time reading my blog.
Much love and respect.
Joseph Ace Tigas | @josephace135
Registered Mechanical Engineer
Reference:
- Hipolito B. Sta. Maria, Thermodynamics
Solutions are being made by me and the sample review problem is found in page 80, problem number 8.
Presentation of equations and formulas were made possible by MS Word 2010 Equations function.
Screenshots were made possible by utilization of Snipping Tool application.
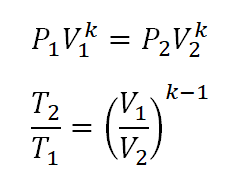
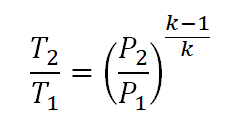
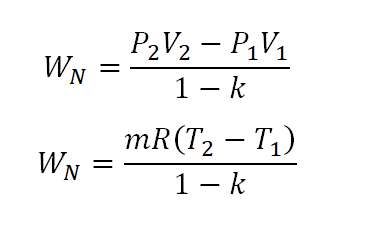
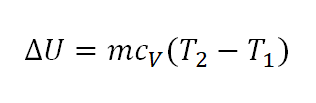
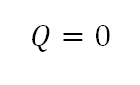
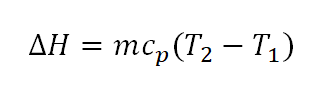
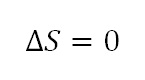
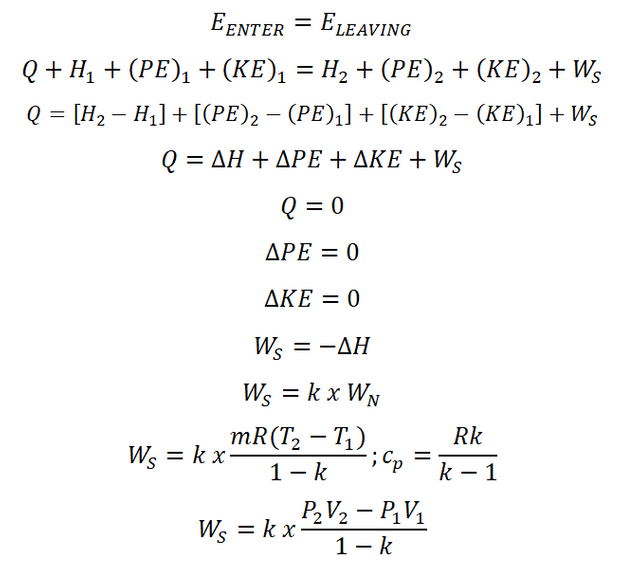
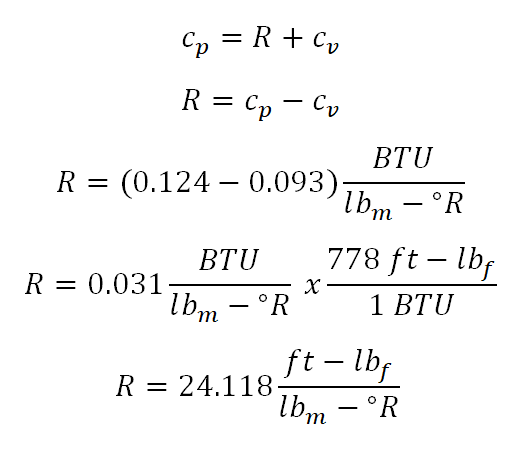
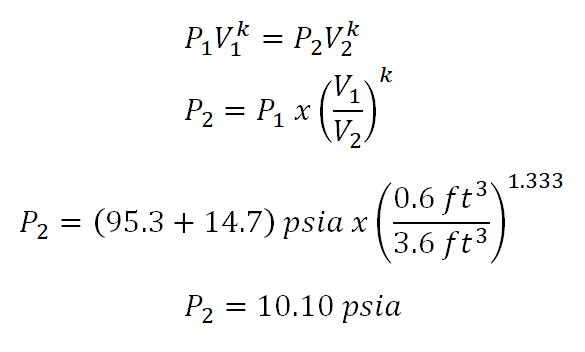
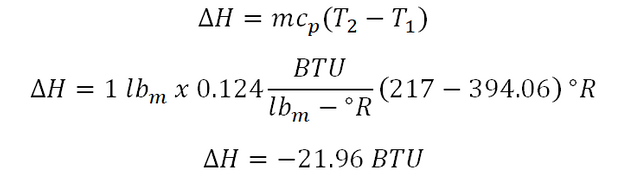
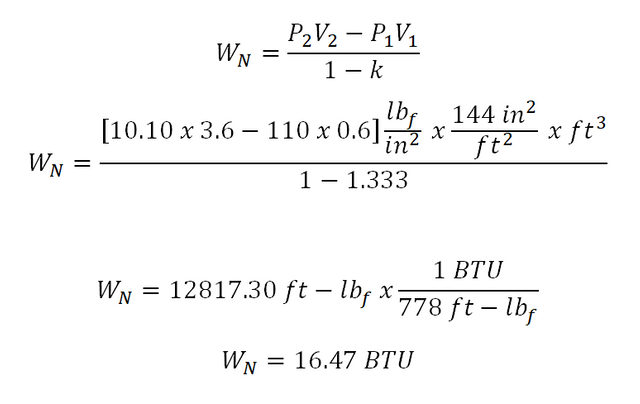
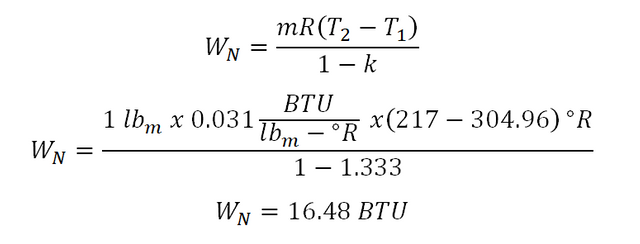
Your blog has received an upvote from the communal account of Steemph.antipolo for being an active discord member and as an active community member. Keep up the good work and best of regards. Keep on Steeming!
You can get a support by joining our discord channel and gain votes from
our curators. Join our discord now
https://discord.gg/7w3hJqw
If you would like to support steemph.antipolo project you can help by delegating your spare SP to us, just click the link below.
50 SP 100 SP 200 SP 300 SP 400 SP 500 SP 1000 SP
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @bitrocker2020, @zord189, @aaronleang, & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by josephace135 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.