Mechanical Engineering Basics 101: The Ideal Diesel Cycle with Sample Problem
Good day everyone!
Previously I made a post wherein I shared my knowledge regarding the Otto Cycle, well today, I will share about my knowledge on the ideal Diesel Cycle.
WHAT IS DIESEL CYCLE?
Diesel Cycle was named after the famous German engineer named Rudolf Diesel who made a great contribution to mankind for his work on the internal combustion engines especially those compression-ignition engines.
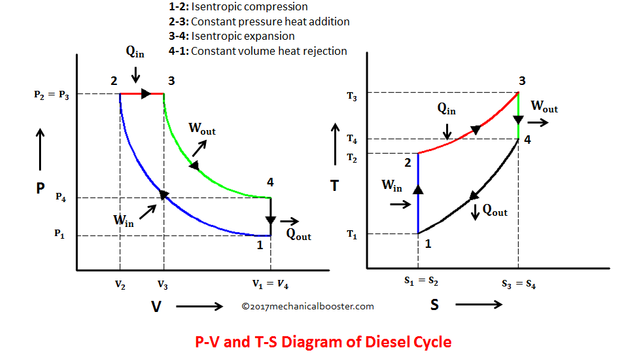
Diesel Cycle is a 4 thermodynamic process cycle wherein it composed of 2 isentropic processes (constant entropy process), an isobaric process (constant pressure process) and an isometric process (constant volume process). These 4 processes are as follows:
- Process 1 – 2: Isentropic Compression (S1 = S2)
- Process 2 – 3: Isobaric Heat Addition (P2 = P3)
- Process 3 – 4: Isentropic Expansion (S3 = S4)
- Process 4 – 1: Isometric Rejection of Heat (V4 = V1)
Wherein S stands for entropy, P stands for pressure and V stands for volume.
Otto and Diesel cycles, differ in the way heat is added on the cycle since in Otto cycle or spark-ignition engines is being added with heat at constant volume whereas for Diesel cycle or compression-ignition engines heat is being added at constant pressure.
The same way as what I have mentioned on my post regarding Otto Cycles, the easy way for understanding the concept of how an ideal Diesel cycle works is by enabling ourselves to be familiar with the P-V (Pressure vs. Volume) and T-S (Temperature vs. Entropy) diagrams, which is shown in the picture below.
In order for us to have a basic knowledge on the performance of the Diesel cycle we are being meet with lots of proven thermodynamic formulas from our professors or even from the books that we read. So here I am going to show the thermodynamic formulas which we need to be familiar for each process in this cycle.
- Process 1 – 2: Isentropic Compression
- This process is also known as the Compression Stroke, wherein the piston of the cylinder rises and compresses the air.
- This process is mathematically proven with these following relations:
- Process 2 – 3: Isobaric Addition of Heat
- This process is also known as the Power Stroke, the cylinder of the piston is being pushed down wherein the compressed air and the oil that is being injected into the cylinder are mixed thereby resulting into an explosion or burning.
- This process is mathematically proven with these following relations:
- Cut-off Ratio (rc) is the ratio between the volume at the end of constant pressure heat addition (V3) and the volume at the end of the isentropic compression (V2).
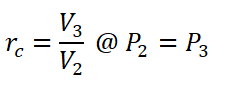
- Pressure (P), Volume (V) and Temperature (T) relations
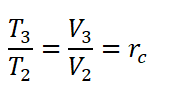
- Heat Addition (QA)
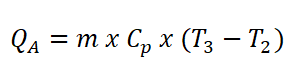
Since there is a hot and cold air standard, which is sometimes between 1.3 to 1.4 value of adiabatic index (k). So here is the formula for the specific heat capacity at constant pressure (CP).
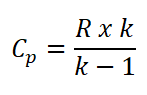
Wherein R is the universal gas constant.
- Cut-off Ratio (rc) is the ratio between the volume at the end of constant pressure heat addition (V3) and the volume at the end of the isentropic compression (V2).
- Process 3 – 4: Isentropic Expansion
- This process is also known as the Exhaust Stroke, in this process the cylinder moves up and the upward force resulted by its movement going up pushes the burnt gases out of the cylinder.
- This process is mathematically proven with these following relations:
- Process 4 – 1: Isometric Rejection of Heat
- This process is also known as the Intake Stroke wherein the piston of the cylinder moves down the cylinder thereby resulting to an intake of air.
- This process is mathematically proven with these following relations:
- Pressure (P), Volume (V) and Temperature (T) relations
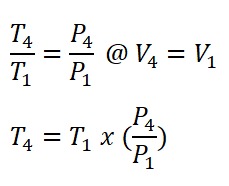
- Heat Rejection (QR)
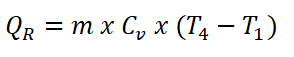
Since there is a hot and cold air standard, which is sometimes between 1.3 to 1.4 value of adiabatic index (k). So here is the formula for the specific heat capacity at constant volume (CV).
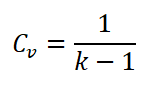
Wherein R is the universal gas constant.
- Pressure (P), Volume (V) and Temperature (T) relations
Now here are the other very important parameters necessary in validating the performance of the Diesel Cycle.
Net Work (WNET)
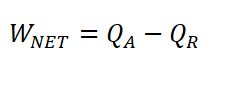
Relations between Compression Ratio (rk)
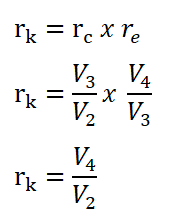
And since V4 is equal to V1. Thus,
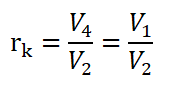
Cycle Effieciency (eDIESEL)
If QA and QR are given we can use the following formula and other derivations.
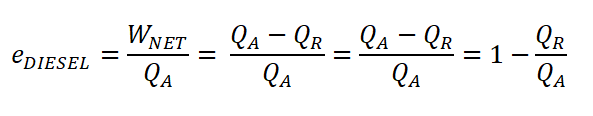
If using the compression ratio (rk) and cut-off ratio (rc) are given, we can use this following derive formula (deriving formula on your own is one of the best ways of mastering the numerous thermodynamic cycles and also for the internal combustion engine cycles, this is my way of learning easily the concept.)
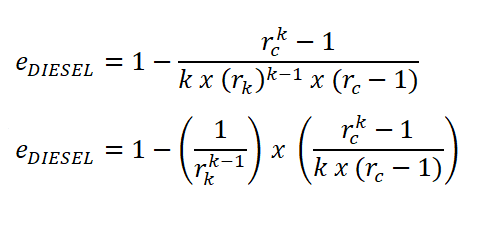
Mean Effective Pressure (PME)
So we are done discussing some of the basic knowledge regarding Diesel Cycles, so let us now proceed to one of the interesting parts in learning the mathematics behind this cycle. So let us solve a sample problem.
In an ideal Diesel engine compression is from 14.7 psia, 80 degree Fahrenheit, 1.43 cubic feet to 500 psia. Then 16 BTU/cycle are added as heat. Make computations for cold-air standard and find:
- Temperature and volume at the end of isentropic compression process (T2 and V2);
- Temperature and volume at the end of isobaric heat addition process (T3 and V3);
- Temperature and pressure at the end of isentropic expansion process (T4 and P4);
- Net work done by the engine (WNET);
- Efficiency (eDIESEL);
- Mean Effective Pressure (PME);
- and lastly, the HP output at 300 cycles per minute.
Note: This problem is an exercise (number 3) obtain from page 114 of the book entitled "Thermodynamics" which was authored by Hipolito Sta. Maria. All solutions and screenshots are made by me in making this article. And as compared from my previous post regarding Otto Cycles I made an improvement in all of my screenshots wherein I added all the units used in each calculation which are all in English units like BTU and lbm.
For this ideal Diesel engine it says that it is using standard cold air, k for cold air is 1.4, wherein k is the specific heat capacity or the adiabatic index of a gas or the ratio of specific heats (CP and CV) or the Poisson constant of a gas. So, we will be using a k of 1.4 for the duration of this sample problem and additionally, the k for hot-air standard is 1.3.
Temperature and volume at the end of isentropic compression process (T2 and V2)
In solving for the value of T2 we need to use the temperature and pressure relation during the isentropic compression process of the ideal Diesel engine.
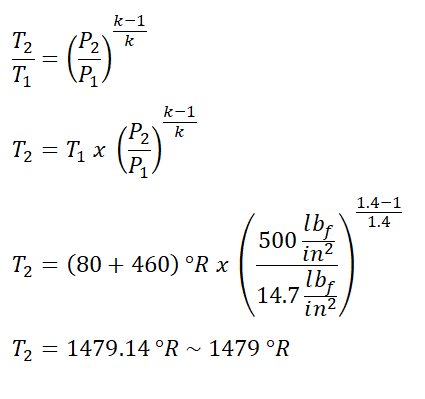
And we obtained a temperature of 1479 degree Rankine for T2.
In obtaining the value of V2 the only thing to do is to use the pressure and volume relation at the isentropic compression process of the ideal Diesel cycle since we aren’t provided of its compression ratio rk.
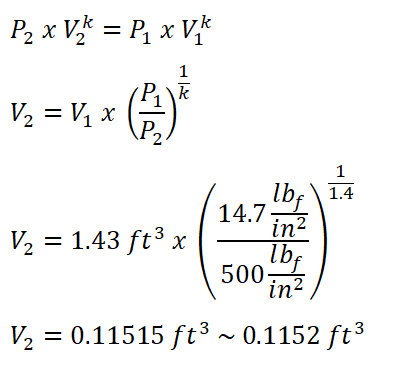
And we obtained a volume of 0.1152 ft3 for V2.
Temperature and volume at the end of isobaric heat addition process (T3 and V3)
In solving for temperature at the end of heat addition process, the first thing to do is to obtain the mass of air (mA) that is entering the engine, which can be obtained by using the universal gas constant formula.
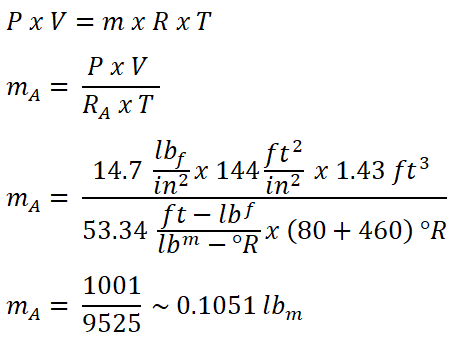
And we obtained a mass of air (mA) which is 0.1051 lbm.
Since we have obtained the mass of air (mA) that is flowing in the engine we can now start solving for temperature at the end of heat addition process, T3, we can obtain it directly by using the formula for the heat added to the cycle at constant pressure (QA) wherein we are provided with the amount of energy added per cycle to the engine and equating the formula so that it now appears to be looking for the T3. And using 0.24 BTU per lbm per degree Rankine as the CP of air at cold-air standard.
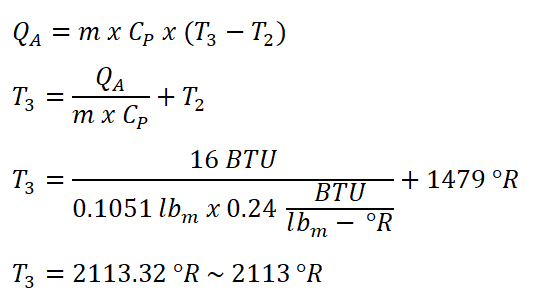
And we have obtained a temperature of 2113 degree Rankine for T3.
In obtaining the volume at the end of heat addition process (V3), we will use the temperature and volume relations during this constant pressure thermodynamic process.
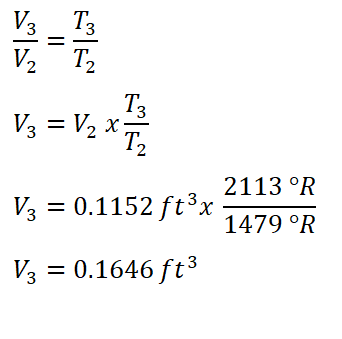
And we have obtained a volume of 0.1646 ft3 for V3.
Temperature and pressure at the end of isentropic expansion process (T4 and P4)
In obtaining for the temperature at the end of isentropic expansion (T4), we only need to use the temperature and volume relations for this isentropic thermodynamic process. Since V4 is just equal to V1 which has a volume of 1.43 ft3.
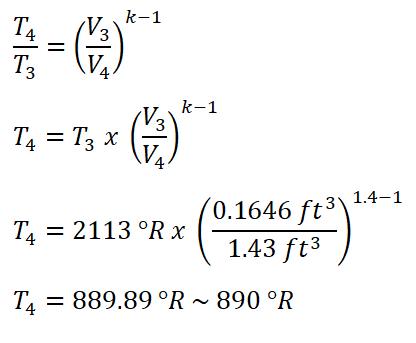
And we have obtained a temperature of 890 degree Rankine for T4.
For the pressure at the end of isentropic expansion (P4), we only need to use the temperature and pressure relation for this isentropic thermodynamic process, wherein pressure at the end of addition of heat process (P3) is equal to 500 psia. And equating the equation to make appear that it is now looking for the pressure at the end of isentropic expansion (P4).
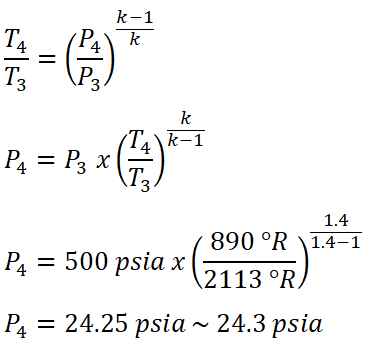
And thus, we have obtained a pressure of 24.3 psia for P4.
Net work done by the engine (WNET)
In obtaining the amount of work done by the Diesel engine, the first thing to do is to obtain the heat rejected during the isometric process, which can be obtained using the formula for the isometric heat rejection. Using a 0.1714 BTU per lbm per degree Rankine as CV of air.
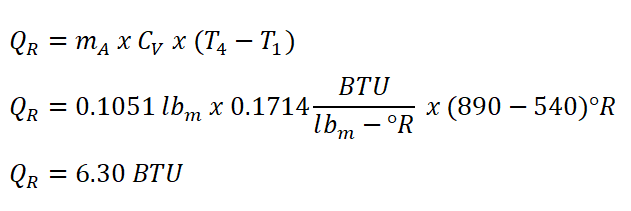
And thus we have obtain an energy of 6.30 BTU for the QR.
Since we have obtained the heat rejected during the isometric heat rejection process, we can now obtain the net work done by the ideal Diesel engine. Using the formula for the net work done, WNET.
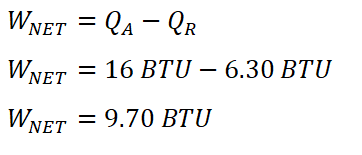
And therefore, the net work (WNET) done by the engine is 9.70 BTU.
Efficiency (eDIESEL)
Since we have obtained previously the amount of energy for both QA and the WNET. We will be using the formula for computing the efficiency, wherein it is using both the QA and WNET as the parameters.
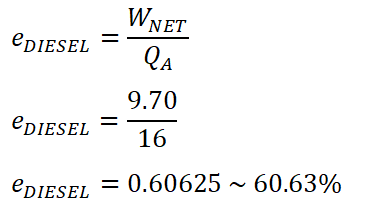
And the cycle efficiency of the ideal Diesel engine is 60.63%.
Interestingly, I will present the other way of obtaining the efficiency using the formula wherein it uses the adiabatic index (k), compression ratio (rk) and the cut-off ratio (rc). Since both rkand rc are not provided, we will obtain these two ratios by using the volumes we have computed previously, specifically, V1, V2 and V3.
First, I obtained the compression ratio (rk).
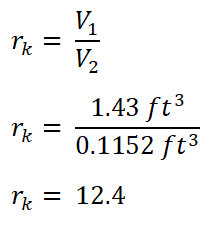
And we obtained an rk of 12.4.
Then, I obtained the cut-off ratio (rc).
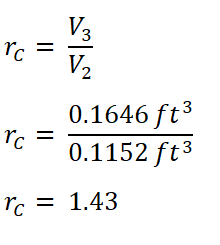
And we obtained an rc of 1.43.
Finally, let's substitute the values we've obtained for rk and rc.
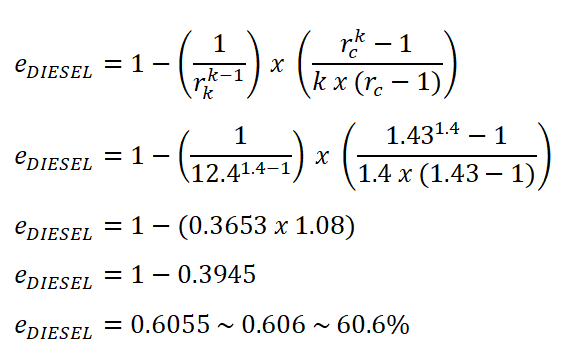
As you can see in the calculation, there is a slight difference since we undergone rounding off of numbers.
Mean Effective Pressure (PME)
In obtaining the mean effective pressure of the ideal Diesel engine, we simply use the formula for the mean effective pressure.
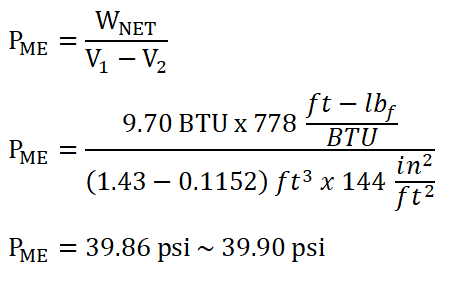
And we have obtained a pressure of 39.90 psi as our mean effective pressure.
Horsepower output at 300 cycles per minute
In obtaining the horsepower output of the ideal Diesel engine, we will just multiply the number of cycles per minute since the units will be reduced into BTU per minute (wherein 1 horsepower (HP) is equal to 42.42 BTU per minute) which is a unit of power, since power is the ratio of energy to time.
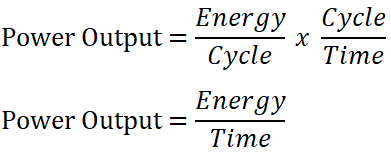
Thus,
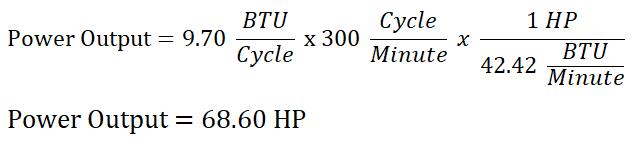
And lastly, we have obtained a horsepower output of 68.60 HP for the ideal Diesel engine.
The same as from my previous post, I would like to advice mechanical engineering students to not memorize the formula, the key to mastering the Diesel cycle is through mastering the concept by way of familiarizing the P-V and T-S Diagram of the Diesel Cycle and mastering the fundamentals of Thermodynamics.
I guess that would be all.
Much love and respect.
Joseph Ace Tigas | @josephace135
Registered Mechanical Engineer (RME)
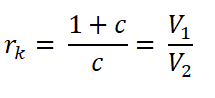
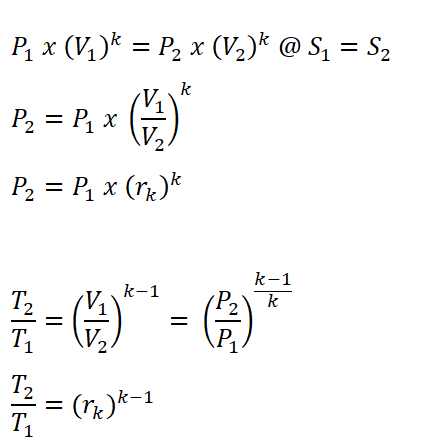
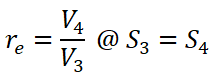
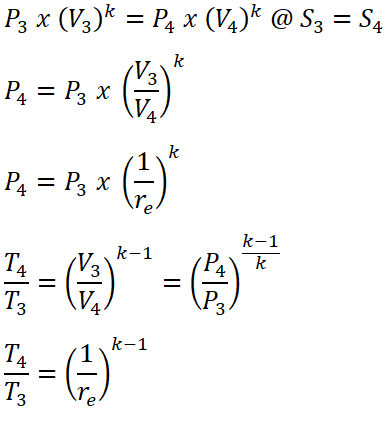
As a follower of @followforupvotes this post has been randomly selected and upvoted! Enjoy your upvote and have a great day!
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
Hi LOVE YOUR POST MAN!!! LIKE MY POST TOO!!!!! HERE IS THE LINK: https://steemit.com/bots/@abusereports/last-minute-upvote-list-2018-05-02
Hi LOVE YOUR POST MAN!!! LIKE MY POST TOO!!!!! HERE IS THE LINK: https://steemit.com/bots/@abusereports/last-minute-upvote-list-2018-05-02
Congratulations! This post has been upvoted by the communal account, @steemph.cebu by josephace135 being run at Teenvestors Cebu (Road to Financial Freedom Channel). This service is exclusive to Steemians following the Steemph.cebu trail at Steemauto. Thank you for following Steemph.cebu curation trail!
Don't forget to join Steem PH Discord Server, our Discord Server for Philippines.
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by The Red Baron from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.