Review on Thermodynamic Processes with Sample Problem (English Units): Isobaric Process
Hi folks!
Since we I have introduced my previous post that there are five thermodynamic processes and these processes are as follows:
- Isometric Process (Constant Volume Process)
- Isobaric Process (Constant Pressure Process)
- Isothermal Process (Constant Temperature Process)
- Isentropic Process (Constant Entropy Process)
- Polytropic Process
So today, allow me to introduce about the constant pressure process which is commonly known to us as the isobaric process. If you’ve wonder under what process those compressors are, well they are the best examples of application of thermodynamic process which holds the pressure as constant. This thermodynamic process is widely regarded as an internally reversible process of a substance such gases or steam wherein it has the capability of going back to its initial state after undergoing some processes.
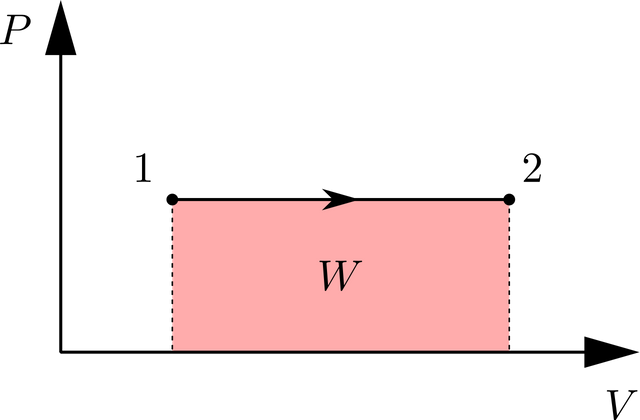
And allow me to proceed on the mathematical formulas that govern the analysis of substances undergoing constant pressure or isobaric process. So here are the formulas and relations:
- Volume (V) and Temperature (T) relation
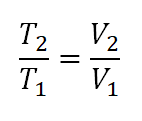
- Non-flow work (WN)
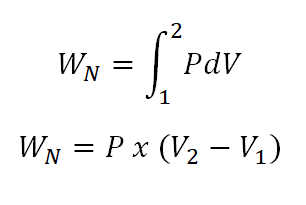
- Change in Internal Energy (delta U)
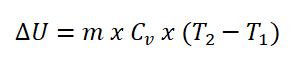
- Heat Transferred (Q)
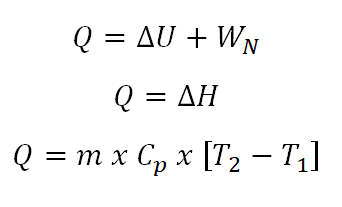
- Change in Enthalpy (delta H)
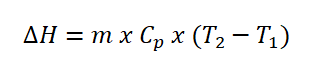
- Change in Entropy (delta S)
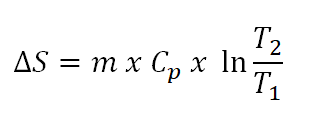
- Steady flow work (Ws)
This formula is derived from the Law of Conservation of Energy wherein it can be expressed as "the energy entering the system is equal to the energy leaving the system".
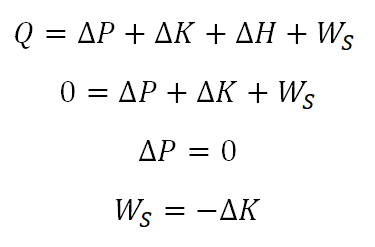
Take note that the delta K is talking about the change in kinetic energy, whereas for the delta P it is talking about the change in potential energy.
So we were able to make ourselves become familiar to those mathematical formulas and equations that all support the performance of the substances under constant pressure process or isobaric. By now let us have a review problem wherein it is about a perfect gas operating under constant pressure process.
Three pounds of a perfect gas with R = 38 ft-lbf per lbm per unit degree Rankine and k = 1.667 have 300 BTU of heat added during the reversible nonflow constant pressure change of state. The initial temperature is 100 degree Fahrenheit. Determine the following:
- Final Temperature (T2)
- Change in Enthalpy (Delta H)
- Non-flow work during change of state (Wn)
- Change in Internal Energy (Delta U)
- Change in Entropy (Delta S)
For this review problem, since we all know that this is under the circumstances of constant pressure process, the first to obtain is the specific heat capacity at constant pressure (Cp) for the perfect gas and we can directly obtain it since we are provided in the problem about the gas constant (R) and the adiabatic index (k) of the perfect gas. And here is the computation for the specific heat capacity of the perfect gas at constant pressure (Cp):
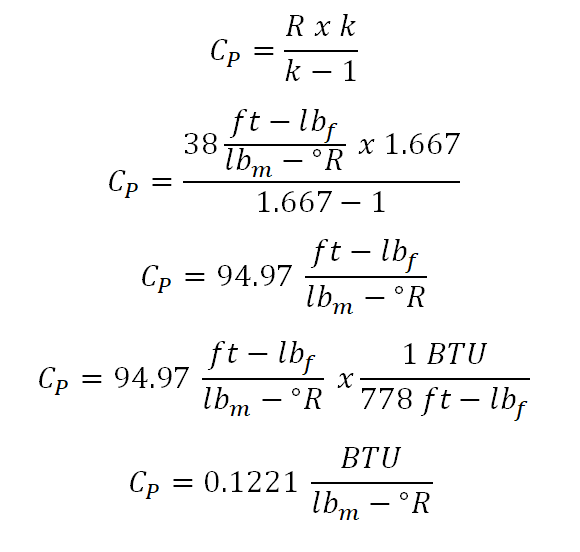
And the calculation being shown in the above photo, the value for the specific heat capacity at constant pressure (Cp) for the perfect gas is 0.1221 BTU per lbm per unit degree Rankine. Since we now have the (Cp), the solutions for obtaining the temperature at final state (T2), change in enthalpy (delta H) and the change in entropy (delta S) would be made possible and straightforward.
So let me start solving on what is ask on the problem.
Final Temperature (T2)
In obtaining for the value of the temperature at the final state (T2) the only in obtaining is to use the equation in computing for the heat (Q) that is being added during the change in state. So the computation goes like this:
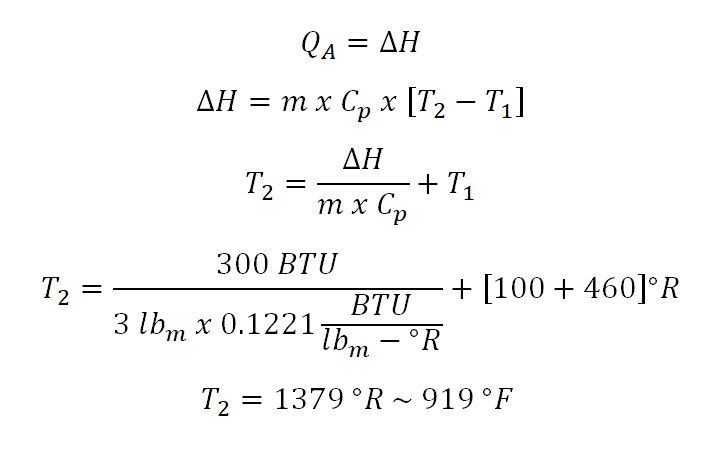
In the above photo, it shows the calculation wherein units BTU and lbm are cancelled out and only the degree Rankine is left. Notice that I always add 460 when it comes to temperatures, it is simply because that the temperature must be in absolute and in English system of units, degree Rankine (R) is the unit of measurement for the absolute temperature which is equal to the numerical value of the degree Fahrenheit (F) with the addition of 460. And thus the temperature at the final state (T2) is equal to 919.67 degree Fahrenheit or 1379.67 degree Rankine.
Change in Enthalpy (Delta H)
For this problem the change in enthalpy is just equal to the heat being added during the change of state of the perfect gas at constant pressure.
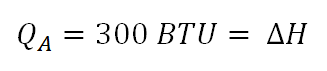
Change in enthalpy is equal to 300 BTU.
Non-flow work during change of state (Wn)
For this portion of the review problem, the easiest way in solving this without obtaining the change in internal energy (delta U)and is achieved by derivation and it is very simple and we will just use the universal gas constant formula in obtaining the non-flow work (Wn) of this perfect gas operating under constant pressure. And the computation goes like this:
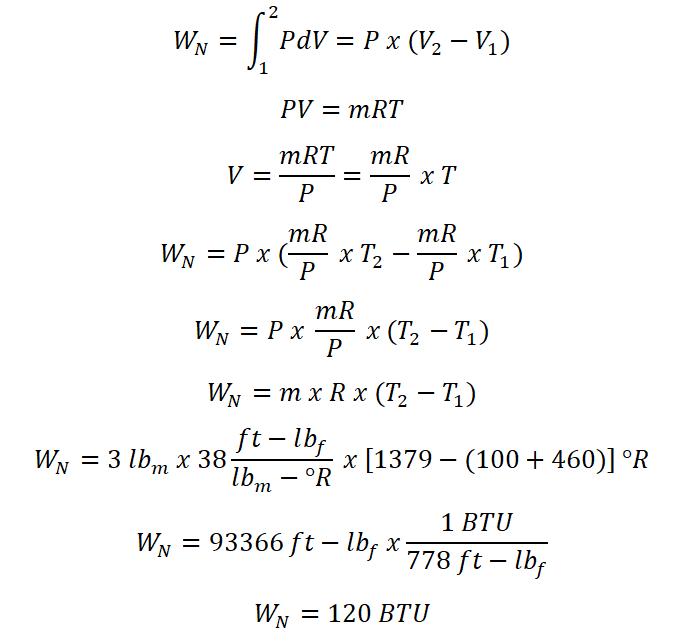
And thus we have found out that the non-flow work (Wn) during the change of state is equal to 120 BTU.
Change in Internal Energy (Delta U)
For the change in internal energy (Delta U) that is being experienced by the perfect gas during the change of state, we can directly obtain it using the formula for computing for the heat transferred at constant pressure. And the computation goes like this:

And thus the change in internal energy being experienced by the perfect gas during the change of state is equal to 180 BTU.
Change in Entropy (Delta S)
As for this portion of the problem, the change in entropy (delta S) can be easily obtained by direct substitution of all the needed parameters especially in this constant pressure process of change in state. And the computation goes like this:
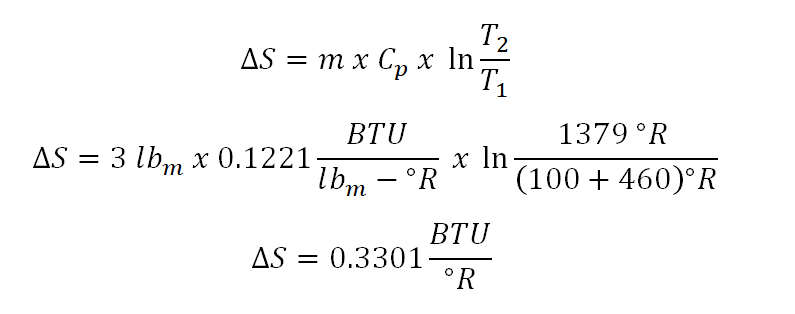
And thus the change in entropy being experienced by the perfect during change of state is equal to 0.3301 BTU per unit degree Rankine.
I guess this ends my blog post relating to the thermodynamic process that is under the circumstances of making the pressure held as constant which is otherwise known as the isobaric process.
Much love and respect.
Ace | @josephace135
Reference:
- Hipolito B. Sta. Maria, Thermodynamics
Solutions are being made by me and the sample review problem is found in page 79, problem number 4.
Presentation of equations and formulas were made possible by MS Word 2010 Equations function.
Screenshots were made possible by utilization of Snipping Tool application.
top notch
sa asa man **** @smaeunabs?
Genius kaau ug posts uy! makaulaw!
Nganong makaulaw man @kyanzieuno? small thing raman ni gud. we are all unique and you've got your own forte too, unleash it.
I know how you have put effort on this engr! Kodus! Keep the fire burning!!!
Thank you sir @morken. I really love solving problems tbh. Btw, congratulations for being curied. You deserve it. Cheers to more curied posts sir, claim it in advance.
Yay! Thank you engr!!! Your next on the line for curie!!! Let’s go!
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @zord189, @aaronleang, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.