Overview on Thermodynamic Processes with Review Problem (English Units): Isothermal Process
Hi folks!
For this blog post and upcoming blog posts most of the topics will be connected to the most interesting subject in the field of Mechanical Engineering and that is Thermodynamics. And I have decided to share the thermodynamic processes and again I will discuss those five thermodynamic processes wherein 1 thermodynamic process and a review problem will be presented in a blog.
So once again here are the five thermodynamics processes:
- Isometric Process (Constant Volume Process)
- Isobaric Process (Constant Pressure Process)
- Isothermal Process (Constant Temperature Process)
- Isentropic Process (Constant Entropy Process)
- Polytropic Process
So today, allow me to share my thoughts and my knowledge about the constant temperature process or widely known to as isothermal process. To mechanical engineers and mechanical engineering students, when we hear about constant temperature process one of the things that come up to our mind is the most famous thermodynamic gas cycle and that is the Carnot Cycle wherein it has two constant temperature processes.
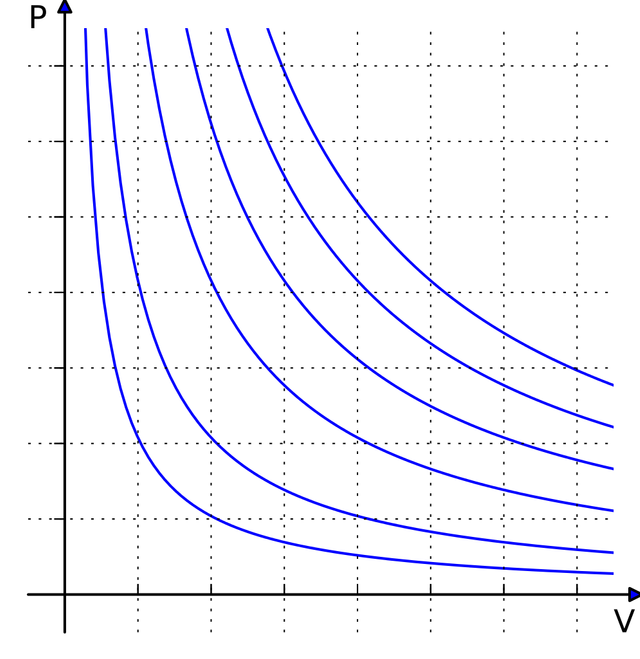
Moving forward, we all know that science would just be a philosophy without the existence of mathematics, since mathematical equations and formulas proved each theorem or law in science. So let us now proceed to the mathematical equations and relations which support the principles of thermodynamic process undergoing a constant temperature.
- Pressure (P) and Volume (V) relation
Since the temperature is held constant, the relation for both pressure (P) and volume (V) follows the Boyle’s law wherein pressure (P) is inversely proportional to the volume (V). And relation goes like this:
- Nonflow Work (WN)
The non-flow work under isothermal process is given with the following formula:
Additionally, there is also an alternative formula wherein in cases of both the pressures of initial and final state are provide.
- Change in Internal Energy (delta U)
Since isothermal process is a constant temperature, therefore the change in temperature is zero and since the change in internal energy is dependent on the temperature, therefore, the change in internal energy (delta U) is zero.
- Heat Transferred (Q)
Based on the equation wherein heat is equal to sum of the change in internal energy (delta U) and the nonflow work (WN). And since the change in internal energy (delta U) for isothermal process is zero, therefore, the heat transferred (Q) is equal to the nonflow work done during the isothermal process. And is given by the following equation:
- Change in Enthalpy (delta H)
Just like the change in internal energy (delta U), the change in enthalpy (delta H) is also temperature dependent and thus it is equal to zero.
- Change in Entropy (delta S)
For isothermal process, the formula for the change in entropy is given by the following equation:
- Steady flow Work (WS)
In obtaining the steady flow work during isothermal process, the basic thing to consider is the law of conservation of energy wherein the energy that enters the system is always equal to the energy that leaves the system. And making the energy balance equation of EENTER is equal to ELEAVING, wherein E is stands for energy. Take note the delta PE stands for the change in potential energy, delta KE stands for change in kinetic energy and delta H stands for the change in enthalpy. So the energy balance goes like this:
So additionally, as what the title of this article say, let me solve a review problem that is in relation to isothermal process and the review problem goes like this:
Four pounds of air gain 0.491 BTU per unit degree Rankine of entropy during a nonflow isothermal process. If P1 = 120 psia and V2= 42.5 ft3, find the following:
- Volume during the initial state (V1),
- Temperature during the initial state (T1),
- Nonflow work done during change of state (WN),
- Heat transferred during change of state (Q), and
- Change in internal energy (delta U).
In solving on what is ask for the review problem, the steps are pretty straightforward since we all have the formulas that govern the performance of isothermal process. So I will now start solving for the parameters being asked for the review problem.
Volume during the initial state (V1),
Since in the above problem, the given are the change in entropy (delta S), the initial pressure (P1) and the final volume (V2) and with that the only way to solve for the volume during the initial state (V1) is by way of using the formula for computing the change in entropy (delta S) and equating it. And the solution goes like this:
During the initial state the volume of air is equal to 7.093 ft3.
Temperature during the initial state (T1),
Since I have just obtained the volume at the initial state (V1), I can now directly solve for the temperature that is constant all throughout the process by using the universal gas constant formula and the solution goes like this:
The temperature that is being experienced by the air is equal to 574.5 degree Rankine.
Nonflow work done during change of state (WN),
For the nonflow work (WN) being exhibited by the air, we can directly obtain it since we all have the important parameters needed in computing for WN, to specific both the pressure and volumes. And the calculation goes like this:
The WN being produced by the air is equal to 282.1 BTU.
Heat transferred during change of state (Q), and
Since the heat transferred (Q) under isothermal process is just equal to nonflow work done by the substance.
The heat transferred by the air at isothermal process is equal to 282.1 BTU.
Change in internal energy (delta U).
Under isothermal process, the change in internal energy (delta U) is always equal to zero.
Well that’s all about my blog post regarding Isothermal Process and I hope you have learned something from this presentation that I made.
Thank you for spending your time reading this blog of mine.
Much love and respect.
Joseph Ace Tigas | @josephace135
Registered Mechanical Engineer
Reference:
- Hipolito B. Sta. Maria, Thermodynamics
Solutions are being made by me and the sample review problem is found in page 79, problem number 6.
Presentation of equations and formulas were made possible by MS Word 2010 Equations function.
Screenshots were made possible by utilization of Snipping Tool application.
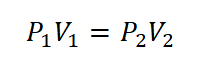
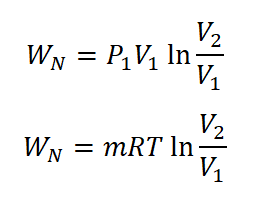
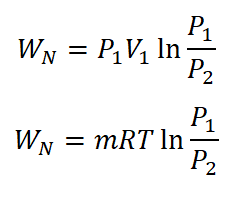
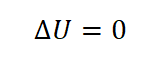
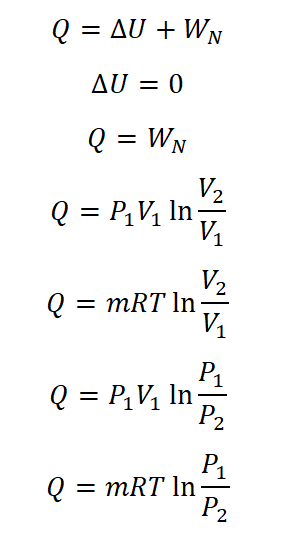
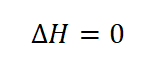
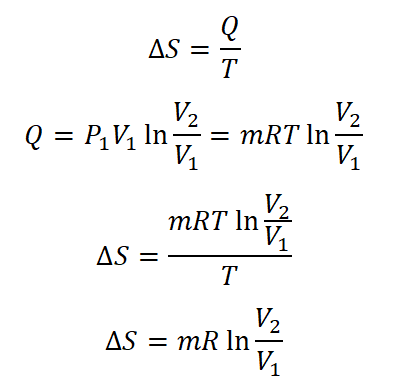
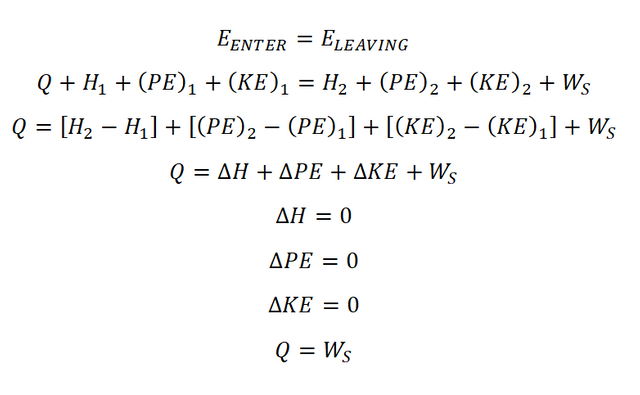
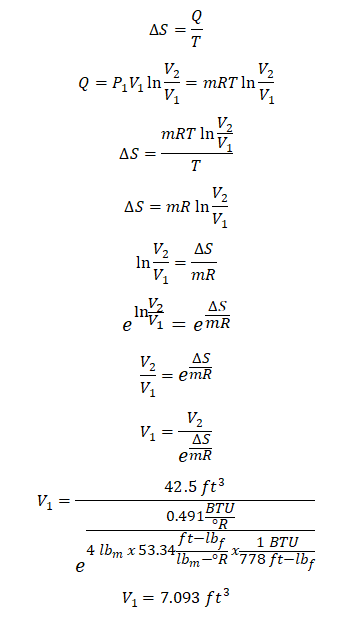
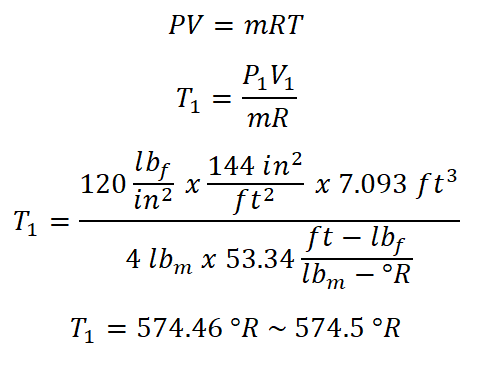
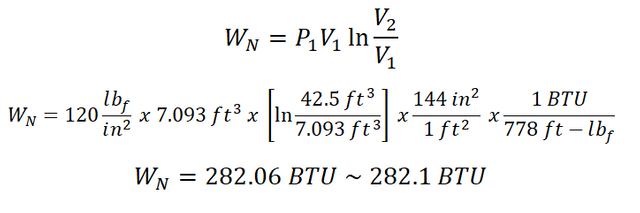
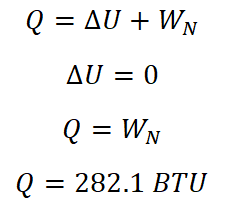
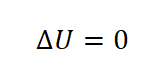
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @zord189, @aaronleang, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
You received an upvote as your post was selected by the Community Support Coalition, courtesy of @steemph.antipolo
@arabsteem @sevenfingers @steemph.antipolo