Using Optogenetics to control the activity of cells (Own research experience)
Hi guys! Today I would like to continue in my series about optogenetics. But! It is going to be a bit different this time. I am going to show you my own experience with this method and will guide you step by step through application of optogenetics. Going to share some results as well. :)
Real science here, get ready!
Optogenetics – quick recap
Optogenetics – a fascinating teqchique that uses light to control cells such as neurons. Light sensitive protein called opsin is being produced by the cell of interest and after light stimulation the cell starts dancing. No. But, the cell does something such as change it membrane potential due to influx of ions. This precise and fast method revolutionized neurobiology as it enables not only to control the activity of neurons in vitro, but also change behaviour of whole animals in vivo. For better understanding check the links at the end of the article- this is like my 7th article on this topic.
Introduction to the experiment
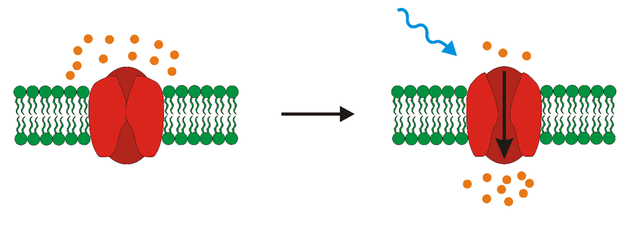
Well, we have these light sensitive proteins called opsins. Light stimulation causes conformational change of opsin, which enables transportation of ions throughout the membrane and thus change in membrane potential (see the picture below). The change of membrane potential may result in either depolarization or hyperpolarization of cells – meaning that you either activate them or silence them. One of these opsins is CatCh which is the variation of channelrhodopsin-2 found in algae Chlamydomonas reinhardtii. It is an ion channel that after stimulation with blue light enables transport of cations into the cell which causes depolarization of the cell. The main difference of CatCh from wild-type channelrhodopsin-2 lies in its significantly increased light-sensitivity and accelerated voltage response – so it is more sensitive and faster (that is a good thing).
So, basically, we wanted to test the properties of CatCh. Human embryonic kidney (HEK ) cells expressing CatCh and whole-cell voltage clamp technique was used. Patch clamp is a technique that enables to measure changes of electric current or voltage which are created as result of ionic flow through membrane (and thus change of membrane potential). In our experimentation we used voltage clamp mode, where the voltage is held constant and current is the measured parameter. Essential component of this method is a glass micropipette, whose tip has a diameter of only several micrometres. Electrode residing inside the pipette is capable to measure even small changes of electric current. To correctly measure these changes the formation of a so called giga seal is necessary. This is achieved by applying positive pressure to form a tight connection between cell membrane and tip of the pipette- so there is no leak current. There are different variations of patch clamp technique and type of the experiments determines which one should be used. We used whole-cell patch that enables the detection of activity of all channels in cell. For more info about this technique read THIS.
First thing to do - Create your GMO cells
First thing we needed to do was to express the CatCh protein in HEK cells. Express means that it is being produced by the cell (more HERE ). There is variety of ways to do so, but we picked lipofectamine transfection that basically uses liposomes (lipid subunits) to put foreign DNA to host cells. How do we know that it worked? Well we used fluorescent tag that was fused with CatCh and subsequently used fluorescence microscope to differentiate between cells that expressed CatCh and those that did not. More about fluorescent proteins HERE.
Meet the experimental setup
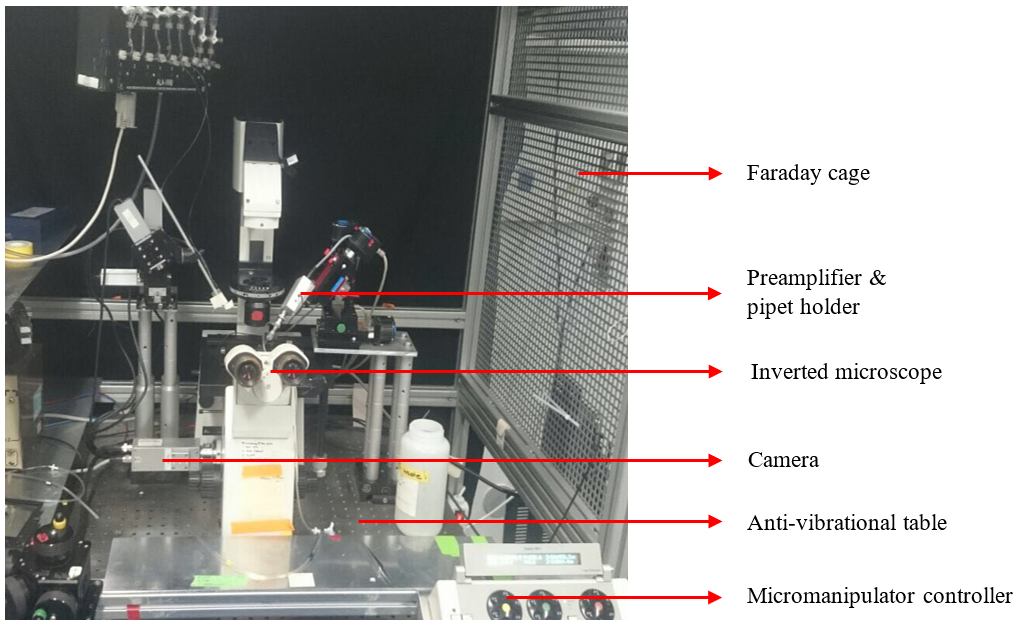
To measure small changes in currents or voltages at the level of single cell, specialized equipment was required. Cells were observed using inverted microscope coupled with computer via camera. Inverted microscope is as it sounds – inverted, so you are not observing the cells from above, but from bottom. Microscope was also connected to a special machine capable of producing different wavelengths of light (because we need only specific light to activate the cells). The microscope was placed in faraday cage to block electromagnetic fields from surrounding equipment. Why is that? Well, the measured currents/voltages were very small and outside interference that is produced naturally by electrical devices could tamper our observations.
Electrical recordings were done using electrode placed in fire polished borosilicate glass pipette filled with internal solution (impedance 1-10 MΩ). I have to tell you that creating these pipettes was hell. They were extremely fragile, and it was not easy to shape them the way you wanted them to be.
Some controls now. To increase or decrease pressure in the pipette syringe and three-way valve were used – this was also used to create the already mentioned giga seal. To amplify measured parameters preamplifier was attached to the electrode. Reference electrode was placed in external solution containing transfected HEK cells. Micromanipulator ensured precise navigation of the pipet- basically a very precise movement controller in all axes. Microscope with micromanipulator were placed on anti-vibrational table. Because you are trying to form a connection between extremely fragile pipette and cell- this is all very delicate, and any vibrations could destroy either cells or pipette tip. Specialized computer software was used to control electrical settings of the electrodes as well as to perform recordings from these electrodes. See the setup for yourselves on the image bellow!
There was also necessity to prepare internal and external solutions. External solution is the one that cells were placed in, whereas internal solution was inside the pipette. Their composition had to be very precise and controlled. But is not important for us now.
Time to do science!
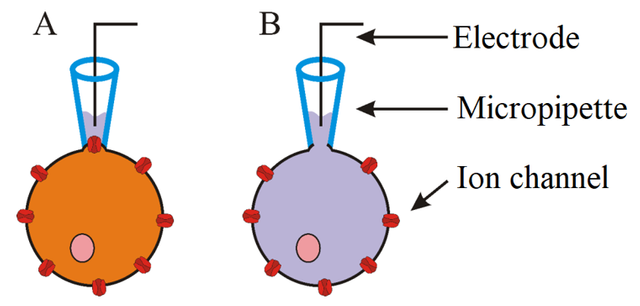
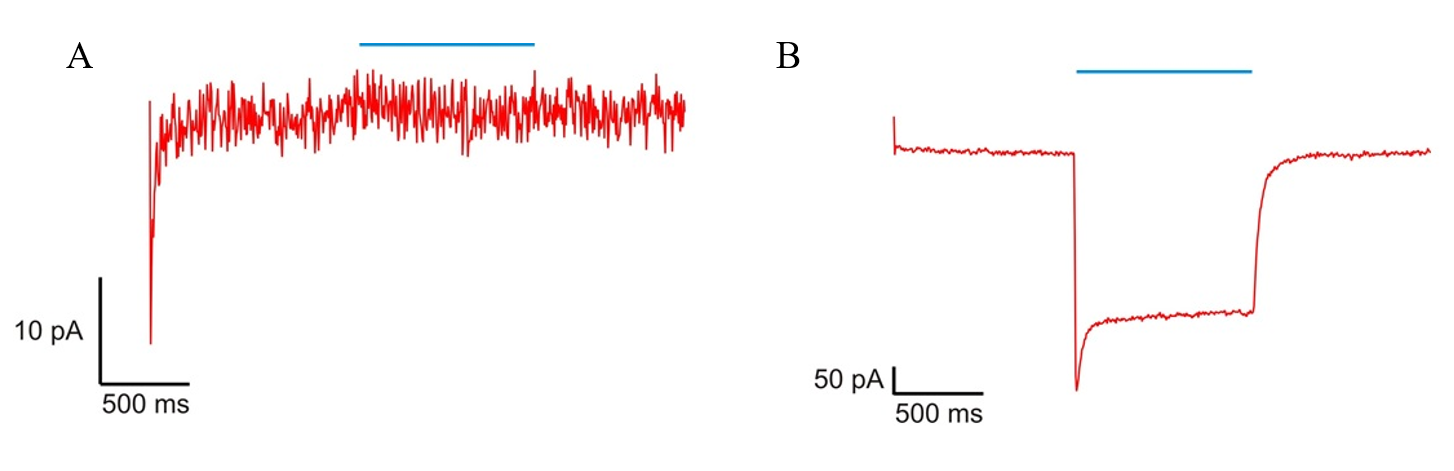
So, you have your cells, you have your experimental setup ready and you want to patch a cell – meaning you want to form a tight connection between the cell and the tip of the pippete. The process was not easy and sometimes it took more than hour to do it. It is also important to note that we measured current, whereas voltage remained constant (voltage-clamp). Well, the initial procedure was to establish giga seal, characterised by a resistance that reaches at least 1 GΩ, as well as whole-cell mode. First changes in measured current were observed after submersion of the tip of the pipette into the external solution. This was due to the change in resistance as voltage remained constant. After approaching cell membrane of selected cell increase in resistance was observed and because of this also a decrease in measured current (basic Ohm's law – current equals voltage divided by resistance). Application of light suction to the pipette resulted in formation of the giga seal. At this point on-cell mode was established (see picture bellow - A). To establish desired whole-cell mode (B) strong suction was applied for short time, stopping after rapid change in electrical current. This change was due to rupture of cell membrane of the cell and thus decrease in resistance, because of the merging of the inside of the cell with internal solution in the pipet.
After establishing whole-cell voltage clamp mode we could begin using light to stimulate the cells. In order to activate the channels (CatCh protein) a short (1 s) light pulse was applied. Cells without expression of CatCh were used as control. Under light stimulus they did not show any change in their current. The light activation of CatCh expressing cells resulted in a negative current change. When the light was removed the current returned to baseline level, but not as fast as during activation. So, what happened? After light stimulation CatCh rapidly opened and thus allowed the influx of sodium cations – this resulted in change in membrane potential and now let’s get back to Ohm's law. The resistance did not change (we have a giga seal, that’s it), voltage was held constant and the measured parameter was current. But we suddenly changed the voltage! Thus, we observed the change in current as well. You can see the change in current on the picture bellow. These results showed us that yes, CatCh can be activated with blue light and can produce rapid change in membrane potential.
We also performed other experiments such as determining the ideal wavelength of light to stimulate the CatCh as well as other experiments to find out more about CatCh. They were lengthy and probably not interesting! :D
Concluding remarks
Allright, I know that this was a bit long, but I really tried to explain everything that I deemed important and in understandable manner. Hopefully you did not die out of boredom and took at least something from this article haha. Next time will be a surprise (because I have no idea), so stay tuned! If you enjoyed reading this, the support me with upvote, comment or just clap your hand now.
Have a great day!
Previous parts of Optogenetics series:
Part 1 – Introduction
Part 2 – History of Optogenetics
Part 3 – Opsins – light sensitive proteins
Part 4 – Gene expression and delivery in Optogenetics
Part 5 – Light stimulation and delivery in Optogenetics
Part 6 – Readout of results in Optogenetics
References
Deisseroth, 2010 - Controlling the brain with light
Kleinlogel, 2012 - Ultra light-sensitive and fast neuronal activation with the Ca²+-permeable channelrhodopsin CatCh
Other references are directly in the text! :)
If the images are not sourced, then they are my own.
Being A SteemStem Member
@jepper : Very nicely written. Resteeming!
Thank you! I am glad you liked it! :)
Nice work @jepper, high quality!
Thank you! :)