How to decipher relevant results in Optogenetics
Hi guys! Today I would like to continue and introduce you to methods that help us read out the outcomes of optogenetic experiments. I will talk a bit about fluorescent dyes, mainly about patch clamp and then a bit about behavioural evaluation.
Quick Recap
Yes, for those of you that have no idea what I am talking about- here is a quick recap. Optogenetics- a relatively new method that combines optics and genetics to control the activity of cells with spatio-temporal precision. It encompasses design and insertion of genes coding light-sensitive proteins called opsins, controlled stimulation of cells (expressing these opsins) with light, but also recording of these cells after light stimulus. This precise and fast method revolutionized neurobiology as it enables not only to control the activity of neurons in vitro, but also change behaviour of whole animals in vivo. Main component of every optogenetic experiment is already mentioned light sensitive protein, termed opsin. Stimulation of opsins with light causes the change in membrane potential of neuronal cells and thus results in activation or inactivation of these neurons.
If you want to know more, then check my previous articles on this topic (links are at the bottom of this article).
Let’s get to it!
The ability to monitor the course of optogenetic experiment is one of the most important parts of optogenetic research. There is necessity of existence of methods capable of localization of opsins in cells or organisms, but also methods that enable to monitor the change of membrane potential after light stimulation. Interpretation of results from these experiments enables us to better describe the properties of opsins, but also helps to optimize experimental conditions.
Fluorescent proteins
Expression of opsins in cells is mostly visualised with fluorescent proteins. Genes coding these proteins are part of the constructs that contain genes for opsins and so this fusion enables the localization of cells for further experiments. Commonly used fluorescent proteins include GFP, YFP or mCherry.
So, what does this mean? I already talked about expression of genes – it means that certain gene product (meaning protein in this case) is produced in certain cells. And when gene for light-sensitive protein is being coexpressed with fluorescent protein it means that the cells that contain light-sensitive protein also contain fluorescent protein. This enables us to find out which cells are the right cells. Because when you are creating these GMO cells (transformation of cells) the efficiency is not 100% for sure. This means that not all cells will express opsins and thus you need a method to differentiate between them. And as these fluorescent proteins emit light (when illuminated with light with correct wavelength), they are the ideal tool for this job.
Patch clamp
Great name, right? To study properties of ion channels, patch clamp methods are most commonly used. Patch clamp enables to measure changes of electric current or voltage which are created as result of ionic flow through membrane and thus change of membrane potential. Essential component of this method is a glass micropipette, whose tip has a diameter of only several micrometres. Electrode residing inside the pipette is capable to measure even small changes of electric current. In order to correctly measure these changes the formation of a so called giga seal is necessary. This is achieved by applying positive pressure to form a tight connection between cell membrane and tip of the pipette- so there is no leak current. There are different variations of patch clamp technique and type of the experiments determines which one should be used. Previously mentioned principle is used in cell-attached patch - this enables to measure activity of one or more ion channels without destroying the integrity of cell membrane. Another variation whole-cell patch enables the detection of activity of all channels in cell. The only difference from cell-attached patch is the application of strong suction after formation of giga seal- that causes tearing of small part of cell membrane and so the inside of the cell is directly connected with the solution and electrode inside the pipette. Variations like inside-out patch and outside-out patch are used for detailed study of function of ion channels, but are rarely used as a part of optogenetic approach.
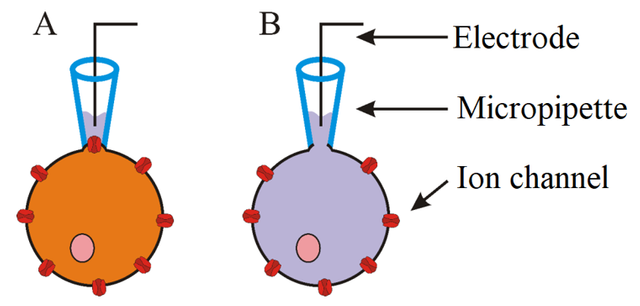
A. – Cell-attached patch. B. – Whole-cell patch.
Behavioural readout
In vivo experiments allow scientists to interpret results through behavioural changes. For example, optogenetic activation of certain brain regions in mouse activated or inhibited aggressive behaviour or motor activity. This method of evaluation is not very quantitative in nature and it is hard to visualize what really happens in the brain. On the other hand, many times the results are quite obvious.
Take a look for yourself - optogenetic stimulation causes motor activity in mice. A bit scary, but fascinating.
Concluding remarks
So, we practically covered all the basic components of optogenetic experiment. Is the series over then? No. Now you are ready to see the really interesting stuff – real experiments! From now the direction of the series is going to be more non-linear and kinda hard to predict, because there are tons of interesting things I would like to show you. Probably I will begin with something I did myself. It is pretty basic, and I also have some results that I can share. So, stay tuned!
And if you have any questions, suggestions, anything, just let me know.
Have a great day! :)
Previous parts of Optogenetics series:
Part 1 – Introduction
Part 2 – History of Optogenetics
Part 3 – Opsins – light sensitive proteins
Part 4 – Gene expression and delivery in Optogenetics
Part 5 – Light stimulation and delivery in Optogenetics
References:
Deisseroth, 2010 - Controlling the brain with light
Deisseroth, 2011 - Optogenetics
Sakmann and Neher, 1984 - Patch clamp techniques for studying ionic channels in excitable membranes
Give visibility to your articles.
Get an upvote 2.5 to 3 times bigger than your send.
Read the explanations on @friends-bot
FRIENDS-MINNOWSBOT is a new bot made for beginners.