Opsins - light-sensitive proteins used in Optogenetics
Hi guys! Welcome to the third part of my series about optogenetics. Today I would like to talk about basic components of optogenetic systems as well as light-sensitive proteins termed opsins that are critical for this technique.
If you missed previous parts, then:
Part 1
Part 2
Basic components of every optogenetic system
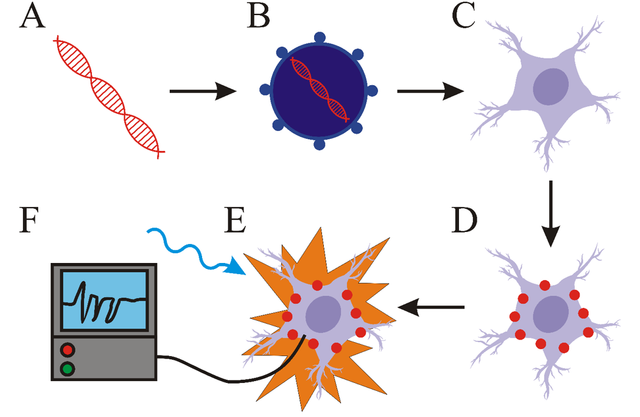
The most important parts of optogenetic systems include light-responsive proteins (opsins for example), a vector and a light source. First step is the creation of a molecular construct carrying a gene for light-responsive protein. This construct is subsequently inserted into a vector (virus for example). Cells transformed with this vector express light-responsive proteins. The next step is light stimulation of transformed cells with light. The wavelength of the light has to correlate with the excitation spectrum of the light-responsive protein. The last step is the detection of the changes in activity of cell (such as the change of membrane potential for example). For this purpose, various electrophysiological methods are most commonly used. Summary of these components can be seen bellow (I drew it, cool right?!).
The construct carrying gene for light-responsive protein (A) is inserted into vector (B) and transported into cells of interest (C). These cells express light-responsive proteins (D). Light stimulation induces change in cell activity (E) which is detected with relevant methods (F).
The most important part – light responsive proteins
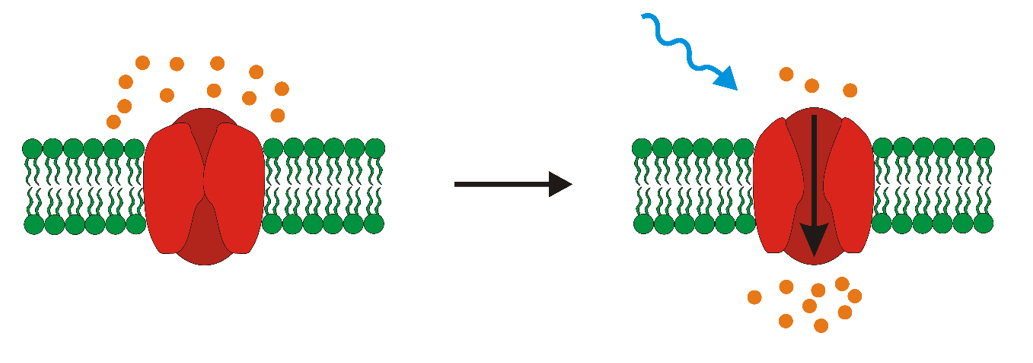
The key component of every optogenetic system is a light-responsive protein that modifies the activity of cell in which it is expressed. Most widely used light-responsive proteins are called opsins.
For opsins to function properly, the presence of retinal is needed. Retinal, a form of vitamin A, forms a functional complex with opsin. Light stimulation of this complex results in photoisomerization of retinal which causes change in conformation of opsin. Mammalian cells have sufficient amounts of retinal already present. But in case of some organisms, for example Caenorhabditis elegans it is required to supply retinal externally.
Opsins can be divided into two categories- microbial and mammalian. Microbial opsins are present in some species of prokaryotes, fungi or algae. They function as ion channels- after stimulation with light they let some ions in or out of the cell. Mammalian opsins can be found in the retina of higher animals, but their mechanism of function is too slow to be properly used for the optogenetic control of cells.
Optogenetics, mainly a neurobiological method, was firstly used to control the activity of neurons in space and time. But why neurons? A neural signal is an electric impulse that is generated by the change of membrane potential on the inner and outer side of cell. The change of this membrane potential is caused by the change of concentrations of ions in intracellular and extracellular space via transport through ion channels. Opsins also function as ion channels. The key difference between neuronal channels and opsins is, that opsins can be precisely controlled with non-invasive method- light. There are generally two effects that can be achieved via the usage of opsins in neurons. The cell can be either activated or inhibited. Activation, or depolarization means that membrane potential has positive values, whereas hyperpolarization, the inhibition of neuronal activity, causes negative values of membrane potential. Thus, opsins can be termed either excitatory or inhibitory.
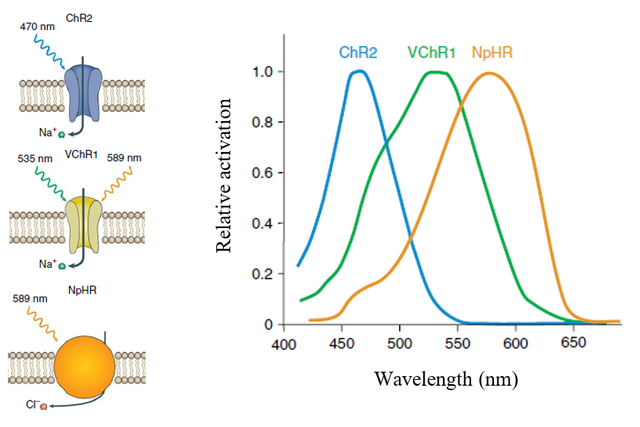
One of the most prominently used excitatory opsin is channelrhodopsin-2 (ChR2) from the algae Chlamydomonas reinhardtii. It functions as a cation channel that, after illumination with blue light, causes the flow of cations into the cell. The other widely used excitatory opsin is channelrhodopsin from algae Volvox carteri (VChR1).
Inhibitory opsins include bacteriorhodpsins or halorhodpsins. Halorhodopsin form Natromonas pharaonic (NpHR) is a chloride pump that transports anions into the cell. This helps N. pharaonic to survive in highly salty environments. NpHR was used to inhibit neuronal function in 2007, but it had several adverse effects. Gradinaru et al. created a modification of NpHR termed eNpHR3.0 that could be efficiently used in cells to cause hyperpolarization. The mechanism of function and excitatory spectrum of some opsins is depicted bellow.
Source
Conclusion
Thank you very much for your time and hopefully you all did learn something new today. Next time we will look at various approaches of gene delivery, so stay tuned!
Bye!
References:
Deisseroth, 2010 - Controlling the brain with light