Thermodynamics Review Problems for Mechanical Engineering Students | Series 26
This is the 26th series of my "Thermodynamics Review Problems for Mechanical Engineering Students". If you've missed the previous series you may try scrolling this blog and head over to the "Curriculum". For this series, I randomly selected a review problem from the Thermodynamics textbook that I’ve been using for years and that review problem is about isometric process. For an overview before solving this review problem, see, Isometric Process.
Review Problem
A
10 ft3 vessel of hydrogen at a pressure of 305 psia is vigorously stirred by paddles until the pressure becomes 400 psia. Determine (a) ΔU and (b) W. No heat is transferred and cv = 2.434 Btu/lbm.Solution
For this review problem, the very first thing to do is to express temperature in terms of pressure and volume since we aren’t provided with the temperatures at the initial and final state of hydrogen gas. This can be done, using the universal gas constant formula which is
PV = mRT, as for me, I’ll be simplifying the equation like this mT = PV / R>. The reason behind that is the formula for the ΔU is expressed as a function of mass (m) and the change in temperature. And additionally, the problem has stated a keyword vessel which we can assume that the process is undertaken by the hydrogen gas is an isometric process or constant volume. Then, we can have a simplified formula for the ΔU which goes like this ΔU = cv * (ΔP*V / R). Since the units provided are in terms of English systems of units, we will be using R = 1545 / MW; wherein MW stands for molecular weight and for hydrogen gas (H2) MW is equal to 2. By using the simplified formula, we’ve found out that the change in internal energy is equal to 431 Btu. Since the problem has clearly stated that there is no heat transfer, thus Q = 0; wherein Q is heat. Finally, we can now compute for the work (W), using the formula, Q = ΔU + W. By doing so, we’ve found out that W is equal to - 431 Btu.mΔT |
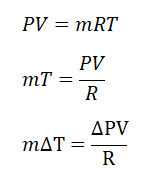 |
ΔU |
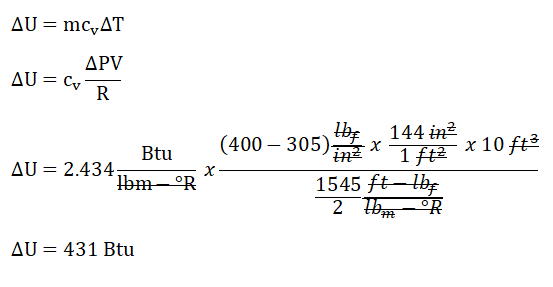 |
W |
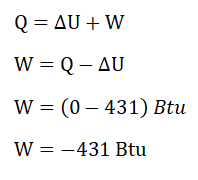 |

Curriculum

Reference
- Sta. Maria, H. [1990] 2012. Thermodynamics 1. Mandaluyong City: National Bookstore Inc..


Brother's great post, good luck always
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by josephace135 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.