Thermodynamics Review Problems for Mechanical Engineering Students | Series 1
Hi Everyone!
Way back in college, having review materials is very beneficial since it lets the students know their level of knowledge whether they need to improve or simply add little bit of speed and accuracy when solving problems related to the specific subjects such as thermodynamics, be it a basic problem or a problem that would cost you lots of hours. As for me and back then, I have lots of compilations of problems in my field of specialization and among the three major subjects for the licensure examination of mechanical engineers my favorite was Power and Industrial Plant Engineering for which Thermodynamics is the basic or the very backbone or say the fundamental subject before proceeding to the complex subjects in Power and Industrial Plant Engineering (P.I.P.E.). It was very unfortunate on my part after the board examination that my laptop got damage and all of my files are there including those review materials, luckily I still have my own sphere of influence to mechanical engineering students from my alma mater and was thankful that @exillediogolvin has his own review materials and without second thoughts he gave me a copy of those review materials. Well, it was a good catch for me since @exillediogolvin is a quizbowler just like me and I know the feeling of being a quizbowler, the need to study lots of topics is a must and to top it all, accuracy and speed must come together to obtain correct answers and make it to the top.
So today, allow me to start the series 1 of my "Thermodynamics Review Problems for Mechanical Engineering Students", I know this is very helpful to them considering that the problems aren't provided with answers and solutions and only provided with choices. But for this case, I won't include the choices to add more challenge to my target audience which is those mechanical engineering students. And with that I'll be computing two review problems which are as follows:
Question 1
A closed rigid container has a volume of 1 cubic meters (m3) and holds air at 344.8 kilo-pascal (kPa) and 273 degree Kelvin. Heat is added until the temperature is 600 degree Kelvin. Determine the change of internal energy.
Solution
For this review problem, we are provided that the ideal gas being used is air and since the change in internal can be increased thru the usage of the following parameters: temperatures (both initial and final), mass (m)and specific heat capacity at constant volume (cv). Since we aren't provided with the mass, we need to obtain it first by using the universal gas constant formula and we are lucky enough that we are provided with the values of the following thermodynamic properties of the air at the initial state which are as follows:
- volume (V1) = 1 m3 (The final volume is the same as the initial volume since this cylinder is undergoing a constant volume process, and is identified that easily thru the keyword
closed rigid container, for when we talk aboutclosed rigid containerit means that the volume is being maintained.) - pressure (P1) = 344.8 kPa
- temperature (T1) = 273 degree Kelvin
Additionally, the universal gas constant (R) of air is equal to 0.287 kilo-joule per kilogram per degree Kelvin. And we can now compute for the mass using the universal gas constant formula; for which the calculation is shown below.
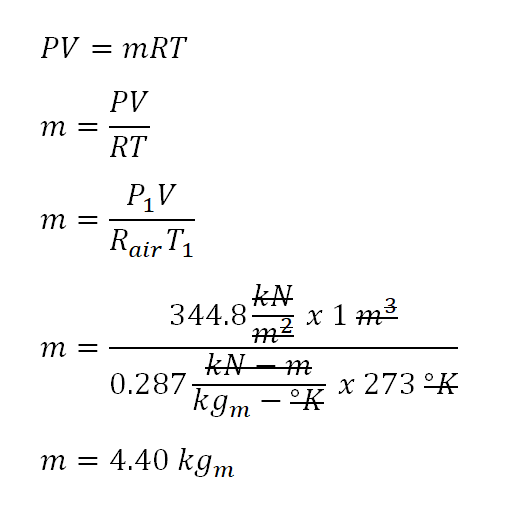
Since we've obtained the mass of the air that is being hold by the close rigid container, we can now directly obtain the change in internal energy that is being exhibited by the closed rigid container when its temperature increased to 600 degree Kelvin. The specific heat capacity of air at constant volume (cv) is equal to 0.7186 kilo-joule per kilogram per degree Rankine. And substituting it to the general formula of the change in internal energy, we've found out that it is equal to 1033.92 kilo-joule or 1034 kilo-joule for which the calculation is shown below.
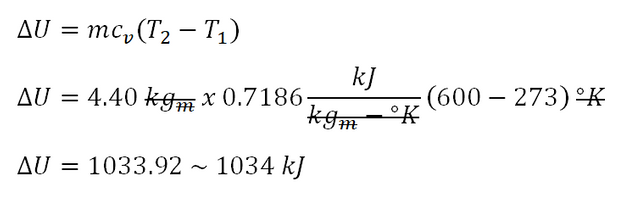
Question 2
A perfect gas has a value of R = 58.8 ft-lbf per lbm per degree Rankine and k = 1.26. If 20 Btu are added to 5 lbm of this gas at constant volume when the initial temperature is 90 degree Fahrenheit, find the change in entropy.
Solution
For this review problem, it states that the perfect gas is undergoing an isometric process or constant volume process. And since it requires that the change in entropy be obtained, the first thermodynamic property to obtain is the the specific heat capacity of the perfect gas at constant volume at k = 1.26. Referring to the screenshot below, we've obtained the specific heat capacity of the perfect gas at constant volume at k = 1.26 is equal to 226.15 ft-lbf per lbm per degree Rankine which is just equal to 0.29 Btu per lbm per degree Rankine.
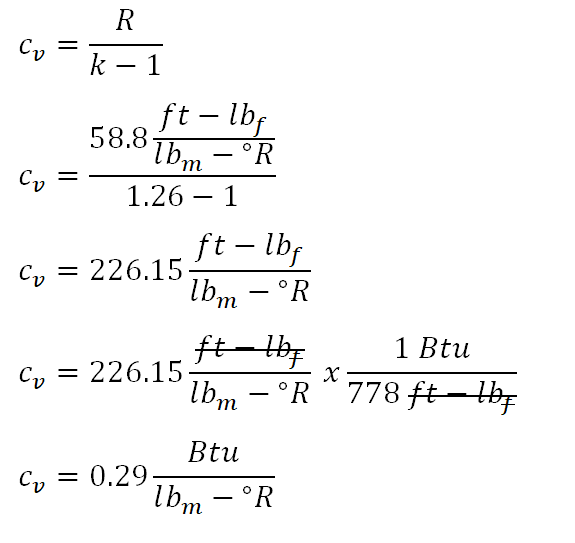
Looking at the given thermodynamic properties it appears that the final temperature isn't provided but the good thing is that we are provided with the amount of heat that is being added to the perfect gas. And the final temperature can be obtained by using the formula used in the previous problem since the heat that is being added to a process wherein volume is held constant is always equal to the change in internal energy. In the screenshot below, we've found out that the final temperature (T2) is equal to 103.79 degree Fahrenheit or 563.79 degree Rankine. You might noticed that in the computation degree Rankine is being paired with degree Fahrenheit the very reason for that is the change in degree Fahrenheit is equal to change in degree Rankine.
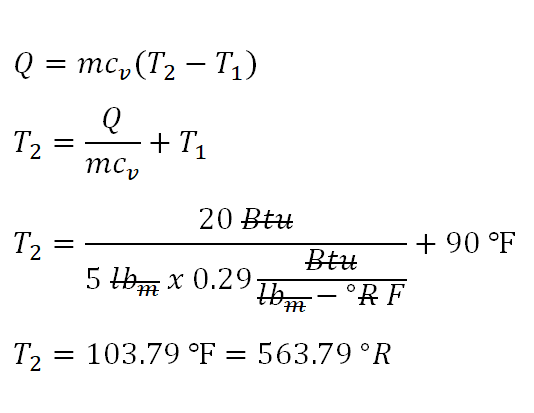
Since we all have the needed parameters (temperatures (initial and final state) and specific heat capacity at constant volume of the perfect gas) in obtaining the change of entropy being exhibited by the perfect gas, we can now obtain it directly as shown in screenshot below. Additionally, by simply looking at the final temperature of the perfect gas, we noticed that it has increased and therefore the expected amount of randomness being experienced by the perfect gas is positive not negative.
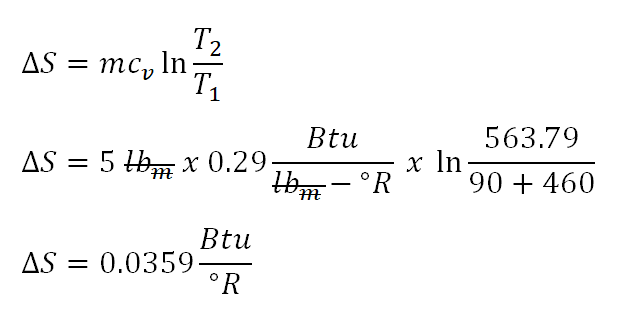
Well, those two review problems ends the series 1 of "Thermodynamics Review Problems for Mechanical Engineering Students".
Thank you and stay tuned for more review problems.
Much love and respect.
Ace | @josephace135

Posted from my blog with SteemPress : https://geuseppedeacenet.000webhostapp.com/2018/07/thermodynamics-review-problems-for-mechanical-engineering-students-series-1
As a follower of @followforupvotes this post has been randomly selected and upvoted! Enjoy your upvote and have a great day!
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by josephace135 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.