Thermodynamics Review Problems for Mechanical Engineering Students | Series 10
This is the 10th series of my "Thermodynamics Review Problems for Mechanical Engineering Students". If you've missed the previous series you may try scrolling this blog and head over to the "Curriculum". This series features two problems relating to isentropic process otherwise known as the reversible adiabatic process. Without much ado, let's get started in solving these review problems.
Review Problem 1
Three cubic feet of oxygen are compressed in a piston-cylinder that undergoes a reversible adiabatic process from a temperature of300 degree Kelvinand a pressure of102 kilopascaluntil the final volume isone-tenth the initial volume. Determine the final temperature.
Solution
For this review problem, the gas that is being used is oxygen gas (O2) and since we were told that this undergoes a reversible adiabatic process which is the isentropic process or in layman’s terms the constant entropy process for which it is heavily dependent on the adiabatic index (k) since this process is mathematically represented with the equation PVk = C wherein P is the absolute pressure, V is the volume, C is constant and lastly, k is the adiabatic index that is always greater than one, (k > 1). For oxygen gas[2], adiabatic index for SI units is equal to 1.395. With that, we can now obtain the final temperature (T2) of the oxygen by using the temperature and volume relations at reversible adiabatic process. And with that, we’ve found out that the final temperature is equal to 744.94 degree Kelvin; as shown in the photo below.
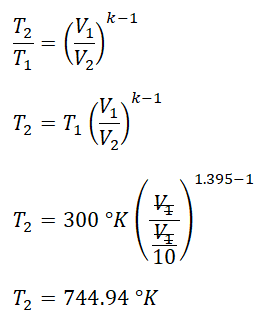
Review Problem 2
In a reversible adiabatic manner,17.6 cubic meters per minuteof air are compressed from277 degree Kelvinand101 kilopascalto700 kilopascal. Determine the change in enthalpy in kilowatts.
Solution
For this review problem, we will use the adiabatic index (k) of air which is equal to 1.4, which is the cool-air standard. In obtaining the change in enthalpy (∆H), we need to obtain first the mass flow rate (ṁ) of air wherein it can be obtain using the universal gas constant formula. By doing so, we’ve found out that the mass flow rate of air is equal to 22.36 kilograms per minute but since the we were asked by the problem to expressed later on the change in enthalpy in kilowatts, since watt is a unit of power which can be simply expressed as a the ratio of energy in joules to time in seconds; thus, the mass flow rate of air is equal to 0.37 kilograms per second.
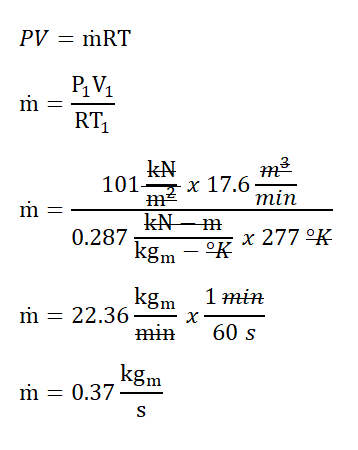
Next to obtain is the final temperature (T2) of the air. The final temperature (T2) can be obtain using the temperature and pressure relations at reversible adiabatic process, for which we’ve found out that it is equal to
481.62 degree Kelvin.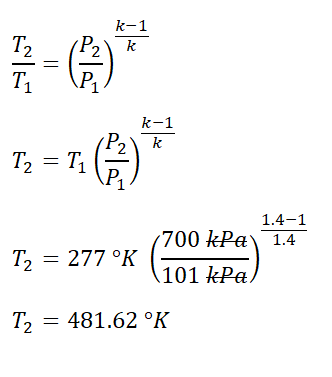
With the necessary thermodynamic properties being obtained, we can now compute for the change in enthalpy (∆H) of the air, wherein it is expressed as the product of the mass flow rate (ṁ), the specific heat capacity of air at constant pressure (cp) and the change in temperature (∆T). With that we’ve found out that the change in enthalpy is equal to
75.71 kilowatts. And as usual, change in degree Celsius is always equal to change in degree Kelvin.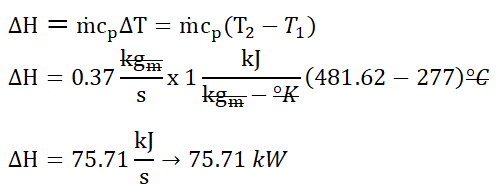
Additionally, for learners that like to input all the thermodynamic properties (starting from the computation of the mass flow rate, then to the computation of final temperature and to the change in enthalpy) in a single equation in their scientific calculators, below is the formula accompanied with the resulting answer.
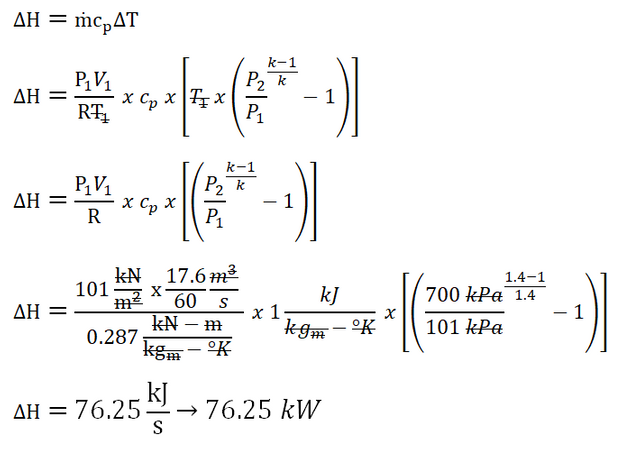
In the above photo, the change in enthalpy is equal to
76.25 kW, whereas in the previous one is equal to 75.71 kW; well, if the mass flow rate (ṁ) of air used in the previous one is 0.373 kilograms per minute, we would arrived with a change of enthalpy that is equal to 76.32 kW. Well, those are relatively close to each other since in the first one we have lots of rounding off numbers whereas in the second one, all was computed at once wherein rounding off of numbers is only done once. Finally, here is a tip for students, if the questionnaire type is of multiple choice, choose what is found in the choices that is close to your computation.
Curriculum

Reference
- PIPE - PROBLEM SET #2 by Alcorcon Engineering Review Center
- Gases - Specific Heat Capacities and Individual Gas Constants. (n.d.). [ebook] Bethlehem, PA 18017: FLSmidth Inc, p.1. Available at: http://catalog.conveyorspneumatic.com/Asset/FLS%20Specific%20Heat%20Capacities%20of%20Gases.pdf [Accessed 19 Jul. 2018].


Very helpful computations right there sir. Keep it Up!
Thank you @jbeguna04, I hope you learnt something. Well, thank you for the design of those GIF photos and dividers, great work you have there. :)
Awesome work @jbeguna04! Great graphic designs.
Great post sir. This would be really helpful for us.
Well thank you @baa.steemit. Stay tuned for more of thermodynamics :)