Electroplating: The Second Best Thing
Alchemy
Before we had Chemistry and other sciences as we now know them, we had Alchemy, a sort of tradition which spread throughout the world, from Europe to Africa and Asia. The practitioners of this tradition worked on converting base metals (such as copper and iron) to more precious metals like Silver or Gold. They also worked on a cure that would eliminate all kinds of diseases and a medication that would serve as an elixir of mortality.. Okay, now relax. I did not say they succeeded, only that they worked on these things. Unfortunately, they did not succeed. However, even though we may not be able to convert one metal to another, we can achieve the next best thing - coat one metal with another, making the coated metal look like the real thing. The process described above is called Electroplating.

All that is gold does not glitter, not all those who wander are lost; the old that is strong does not wither, deep roots are not reached by the frost. J. R. R. Tolkien[1]
Electroplating
Electroplating is the process of passing electric current through a solution or molten substance called an electrolyte which is usually a salt. Two conductors called electrodes are dipped in the electrolyte and connected up to a source of direct current such that when electric current is passed through the electrolyte , it is decomposed into a metal and another substance and some of the metal atoms are deposited on one of the electrodes. The above explanation is very crude but we have to do with it for now.
Whenever you set out to do something, something else must be done first.[3] - An extension of Murphy's Law
Electrolysis
Electroplating is similar to electrolysis so it would be worth our while first to familiarise ourselves with Electrolysis. Even though other scientists, starting from 1785 had worked on the process of electrolysis and used it to achieve their various aims, it was Michael Faraday who coined the word from two Greek words.
Just as earlier described, electrolysis involves the passing of a direct current (DC) through a molten salt or salt solution (electrolyte) and producing chemical reactions at the electrodes which are made of conducting materials. Electrolysis is set up using the following components:
Two Electrodes: these are electrical conductors that provide the physical interface between the electrolyte and the electric circuit that provides the energy. One is called the anode(connected to the positive terminal of the direct current voltage source). The other electrode is the cathode (connected to the negative terminal of the direct current source). The electrodes are usually metals, semiconductors or graphite.
Electrolyte: this is a substance, usually a soluble salt or a polymer that is capable of producing mobile ions that can conduct electric current, thereby completing the circuit.
Direct Current (DC) Supply: this is usually a battery which is capable of creating a potential difference between the electrodes and providing the energy required to ionise the electrolyte to enable the freeing of electrons that will carry current to the external circuit.
Why go through all this trouble? Well, electrolysis is vital in the separation of elements such as the process of separating a metal from its ores.The process of electrolysis merely is the interchange of atoms and ions by the removal or addition of electrons from the external circuit. In other words, when the circuit is closed, current flows because electrons from the battery cell flow into the electrolyte through the anode (positive terminal). This is where oxidation takes place.
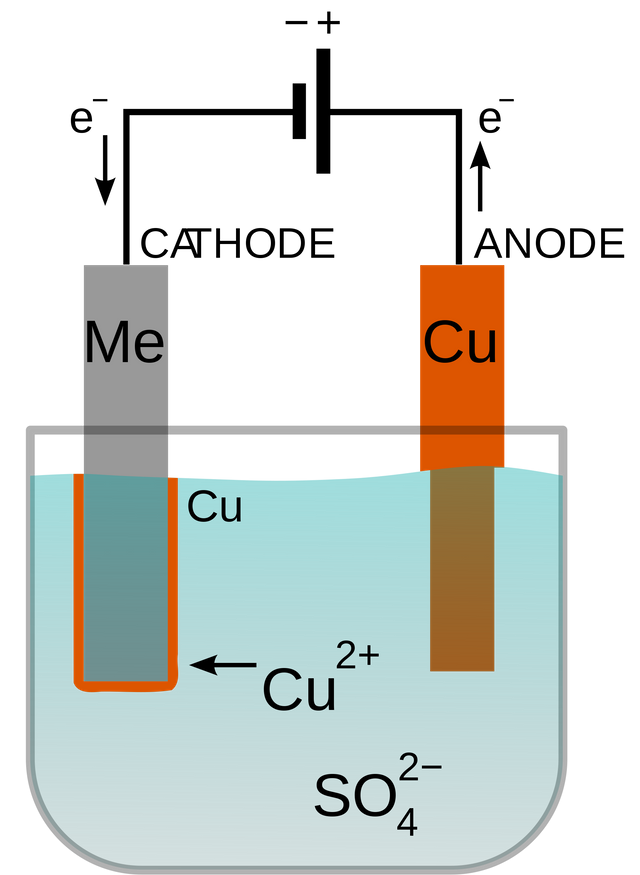
For instance, in the electrolysis of copper (II) sulphate (CuSO4) using a copper anode and a graphite cathode, the following reduction reaction happens at the cathode:
Cu2+(aq) + 2e- → Cu(s)
whereas, at the anode, there is an oxidation reaction as follows:
2H2O(l) → O2(g) + 4H+(aq) + 4e-
By the way, in Chemistry, reduction means that the atom or ion has gained electrons whereas oxidation means that the ion or atom has lost electrons. Each electrode as described above attracts ions that are of the opposite charge. In this case, the positively charged copper ions (cations) would migrate towards the electron-providing negatively charged cathode where it would be deposited as a thin film of copper. The negatively charged sulphate ions, SO42- (anions) move towards the electron-extracting, positively charged anode. At the anode, they will contribute electrons to the copper used in the electrode, forming CuSO4 which enters the electrolyte ensuring that the solution remains of the same concentration at the end of the process.
When a positively charged ion gains electrons and becomes neutral, it is said to have been discharged. In this process, when the positively charged copper ion receives electrons at the graphite cathode, it becomes a neutral copper atom which must leave the electrolyte and be deposited on the graphite cathode. The process now described is called Electroplating or Electrowinning. It is also applied in the refining of metals, in which case, the process is called Electrorefining.
Electroplating Plastics or Non-Conductors
You may have seen some plastics with a metallic finish. Those are probably electroplated plastics. The thing is that plastic is cheap and quite commonplace. Because of these two properties, it is unsuitable for luxury goods unless someone figured out a way to make the plastics look like something else, perhaps silver or gold. This is what is achieved through electroplating plastics.
Ordinarily, because plastics are non-conductors, it is easy to conclude that they cannot be electroplated. However, if they undergo specific treatment, they can be electroplated just like metals. The prerequisite treatment for plastic before electroplating are cleaning thoroughly and removing any rough surfaces, etched with an acid solution and treating it with a catalyst to help speed up the chemical reaction. Finally, the plastic is dipped in a copper or nickel solution such that the plastic is covered by a fragile layer of the conducting material to enable it to conduct electricity.
Conclusion
Electroplating is a redox reaction which means reduction-oxidation reaction. Reduction happens at the cathode where a cation gains electrons and oxidation happens at the anode where anions give up or lose electrons to form the electrolyte, thereby restoring the concentration of the electrolyte to its original level. A naturally occurring slow oxidation reaction is rusting or corrosion.
Base metals (usually cheaper) can be protected from corrosion or made more beautiful by coating them with precious metals (which are at the same time more inert and more expensive), through the process of electroplating. In other words, two main reasons for electroplating are 1. to beautify and 2. to protect the base metal from corrosion. Plastics can also be electroplated to make it appear more luxurious and beautiful than ordinary plastic. The non-conductivity problem that electroplating plastics presents is bypassed by cleaning and dipping the plastic material in a conductive liquid before electroplating.
Thank you for visiting my blog.

Being A SteemStem Member
Very nice topic of discuss. It connects to me in a very nice way. I would like to share my knowledge here.
As much as electroplating is a good way of applying electrolysis, corrosion is a bad way it happens.
The metal which is corroding is the anode, another part of the metal (or something which is electrically connected to the metal) is the cathode and the corrosive environment (soil or atmosphere) being the electrolyte. With all this present, the electrolytic (or corrosion) cell is complete and the metal corrodes with time.
This process can be prevented by a series of means of which the most efficient being cathodic protection
I love the way you simplified the whole process using corrosion. It makes it easy to remember how electrolysis works without having to think too much. It seems I have entered your domain this time. Thank you for your valuable comment. I have learned something from it.
Thank you for your nice comment.
I would have written more but i was dozing at that time. A lot of these concepts are guided by the seemingly simple and fundamental principles (transfer of electrons and the likes).
I am glad you are interested in corrosion,it is an interesting aspect of engineering (I'm actually a mechanical engineering graduate)
Good morning
What I know Chemistry in the future is growing very rapidly and is known to many people. But, few know who the real first person who invented the exact sciences.At Abu Musa Jabir Ibn Hayyan (721-815 H), the first Muslim scientist to find and introduce the discipline of your chemistry. Born in the city of classical Islamic civilization, Kuffah (Iraq), this Muslim scientist is better known as Ibn Hayyan. While in the West he is known by the name of Ibn Geber.
His father, a drug dealer, died as a 'martyr' spreading the teachings of Shia'a. Jabir received his education from the king of the Umayyads, Khalid Ibn Yazid Ibn Muawiyah, and the famous priest, Jakfar Sadiq.Ia also studied at Barmaki Vizier during the Abbasid Caliphate of Harun Al Rasyid. It was found by Jabir proving, in the past is not only cunning in the religious sciences, but also the science of the sciences.
"After medical science, astronomy, and mathematics, the Arabs made their greatest contribution to chemistry," wrote the Western historian Philip K Hitti, in Arabic History. Jabir was dubbed the Father of Modern Chemistry.
Thaks you.....
Wow, I did not know all that. Thanks for sharing. The truth is that no other group of people would tell your story unless you first started telling it loudly enough that it drowns out other people's voices until they start telling your story.
Thank you for sharing how the Arabs contributed to Chemistry and thank you for visiting my blog as usual. You're appreciated.
This is actually possible, when acting on the nuclear structure of the atoms. But this is not chemistry anymore :D
Hell yeah, It's not chemistry, It's Heavy metals and nuclear fusion stuff. :P also nuclear fission
Thank you for pointing that out. I really hadn't thought of it, maybe because I was drowsy by the time I finished the post. But what would it require in terms of energy to fuse two non-radioactive metals?
Thank you for visiting my blog. I have followed you.
Yes, you are right. I didn't think about altering the nuclear structure of atoms. But I wonder, what would be the cost implication of that using today's technology? Maybe we are better off just buying the precious metal or is it cheap. I have limited knowledge of that field.
It is my greatest honour to have you visit my blog. I'm moving up in the steemit world :)
It will be more expensive than the gain, for sure :)
I thought as much. Thanks for the reply.
How efficient is the production of hydrogen using electrolysis?
Using electrolyser to produce hydrogen through electrolysis is only about 75% efficient. If we use green alternatives like solar energy to produce the electric power, we can get a 10% efficiency from the panels, making the process even less efficient. I think this is one of the biggest problems of powering cars with hydrogen. Thanks for your comment.
Another good article explaining Electroplating and Electrolysis, combining Chemistry and Electricity, two of my most favourite subjects. Interestingly enough, I never study those two topics during my school days. Thanks for providing more info. Upvoted!
Thanks Bro. You are right. The last time I studied this was in secondary school. Thanks for your support.
Being A SteemStem Member
Nice post sir, u will make a good teacher. Hope to here more from you next time
Thank you for the compliment. I love teaching. Thank you for coming around.
Science is just great. This is one of the best articles I have read from your blog. I agree with you on this;
Good topic, Good explanation. Well done!
Thanks Bro.
This was a really good and educating read.
It took me right back to my chemistry days.
So, it's safe to say that electrolysis is the method/process through which electroplating is achieved?
Thanks for sharing.
😊
Yes dear. Thanks a lot.