Chemistry Lesson: Part 10 (Atomic Structure Part 1)
Original Image Source
Introduction
This series of posts seeks to present the material covered in the first semester of a college level general chemistry course, in an easily digestible steemit blog post format. The series is intended to be read, and experienced in sequential order starting with Post 1. The material will build upon itself, and potential exercises included (problem sets), will pertain to the post they are contained in, or any previous post. Each post will pick up immediately where the previous in the series left off. Please check out the #chemistry-lesson tag for all posts in this series. I hope you find this series to be informative and beneficial toward your understanding of chemistry and science in general.
Immediate Preceding Post
Part 9: Gasses and Reactions in the Gas Phase 2
Legend for This Section
Yay! Apparentlysub scripts function now! Great work Steemit dev team!
A History of the Science of Understanding The Atom
Lets Begin with Electromagnetic Radiation
Electromagnetic radiation is a form of energy that exists as both a particle and a wave, it exists in many forms, all of which move through a vacuum (like space for instance) at a speed of 3.0 x 108 meters/second. We refer to this speed as the “speed of light” but it’s really the speed of any form of electromagnetic radiation (of which visible light is but one form). When we think about electromagnetic radiation from the perspective of it acting as a wave we must think about things like wavelength (λ) which can be thought of as the distance between two identical points on waves in succession (like the distance between the peak of one wave and the peak of the very next wave). Speed (u) and frequency (v) which is the number of waves which pass a particular point in a defined amount of time.
We can relate some of these properties together as well: λ x v = u. Or the wavelength (distance of one wave) multiplied by the frequency (number of waves passing by a point in a defined amount of time) is equal to the speed the wave travels (aka the distance traveled per a defined amount of time). For electromagnetic waves we know what the speed is, we already defined it its 3.0 x 108, so we can use the equation above to figure out other properties about electromagnetic waves.
Let’s say I am an asshole (You’re nodding right now… yeah you’re an asshole @justtryme90 that’s for sure!) and I shine a green laser pointer right in your eye:
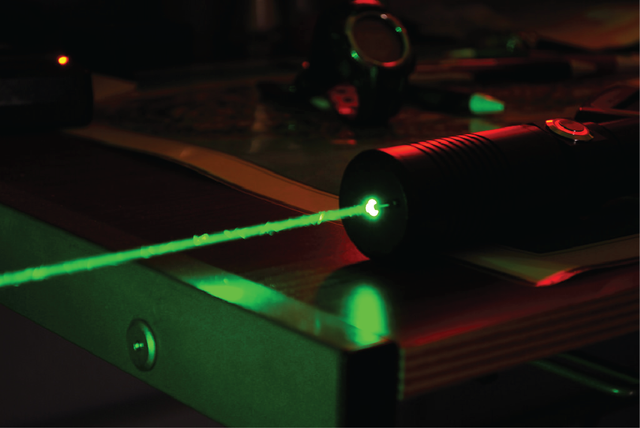
Before you get up to kick my ass you first ponder … what is the frequency of this light that is blinding me right now? Well thanks to the above equations and knowledge that the light from a green laser pointer has a wavelength of 532 nm or (532 x 10-9 m). We know that:
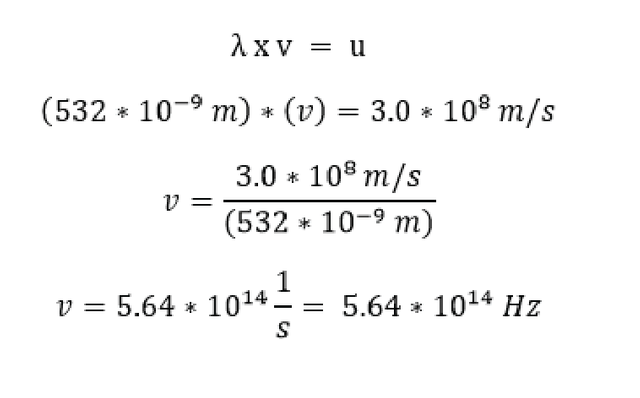
Knowing the wavelength and speed of light we can easily convert to the frequency of the light (v) we just divide the speed by the wavelength, this gives us a number with the unit 1/s (5.64 x 1014) that unit 1/s is called Hertz or (Hz) which actually refers to the number of cycles per second. In our case a cycle is one wave, so when that laser is blasting your eye, you are getting hit by 5640000000000000 waves per second. (haha!! Deal with my laser!!!)
Anyway, so at this point you are sitting here and likely thinking… why are you prattling on about waves and lasers for anyway, what does this have to do with the atom? This will all make sense, I promise you, keep reading!
Max Planck, (early) Quantum Theory And That Einstein Guy
Whenever we heat a solid, it emits radiation, these emissions occur over a variety of wavelengths depending on the amount of heat and the material which is being heated. In the late 1800’s researchers determined that the amount of energy radiated from these heated solids, depends on the wavelength of the emitted energy. It was Max Planck that figured out how to quantify the energy released. He said that atoms and molecules were only capable of emitting specific amounts of energy in small packets called quantum and defined the energy of a single quantum by the following equation: E = hv. Where E is energy, h is planck's constant or 6.63 x 10-34 J s (J is joules, and s is seconds), and of course you already know v is frequency of the radiation emitted.
Planck was buddies with another famous Scientist from the late 1800’s that you may be familiar with. Albert Einstein. Soon after learning about Planck’s theory Einstein came up with the photoelectric effect and said that for light these quantum’s of energy defined by hv (from the equation above), were called photons, and he used Planck’s equation to define the energy of the photon. He also thought that a beam of light was like a stream of these photon particles, and that each particle had Energy as defined by Plancks equation above for the quantums.
Einstein continued in his brilliant ways and deduced that since electrons are held by metals through attractive forces, they should be able to be removed by hitting the metal with enough energy, and in this case the source for that energy could be a beam of light (aka a beam of energetic photons)! Einstein thought that if the frequency of light that was shined onto a metal surface was equal to the binding energy of an electron that it would break the electron loose. He also took things a step further and said if the energy of the light was greater than the binding energy of an electron, it would not only break loose, but also gain kinetic energy and move!
So there you have it, light can behave like a wave, but also behave like a particle. Now I imagine you’re still sitting around going... damn it @justtryme90 get to the atoms already, this post is getting long and you have just been yammering on about history and light beams and lasers in my eye!! Patience young Padawan I say to you. The Atoms are coming (and will be continued in the next post in this series!) why I have been telling you all of this will make sense shortly!
Niels Bohr and the Theory of the Hydrogen Atom
For a long time scientists had been studying the Emission Spectrum of things. The emission spectrum is the spectrum of radiation that is produced from a substance (so all of the different wavelengths, and they generated it by heating things up!) When gaseous atoms are heated up they emit light at specific wavelengths, these emission spectrum are referred to as line spectrum as only specific lines in the electromagnetic spectrum show up brightly. Soon after Planck and Einstein were collectively figuring out how light is both a particle and a wave, a different physicist named Niels Bohr came up with an explanation for the emission spectrum that came from gaseous Hydrogen. Most of that explanation was wrong (sorry Niels LOL rekt) but part of it was less wrong (sort of!!!!)! At the time of Neils Bohr researchers knew that atoms contained electrons and protons, and they thought of atoms as nuclei with electrons rotating around in discreet circular orbits, kind of like planets around the sun (we know this isn’t correct, and we will get to that in the next post!).
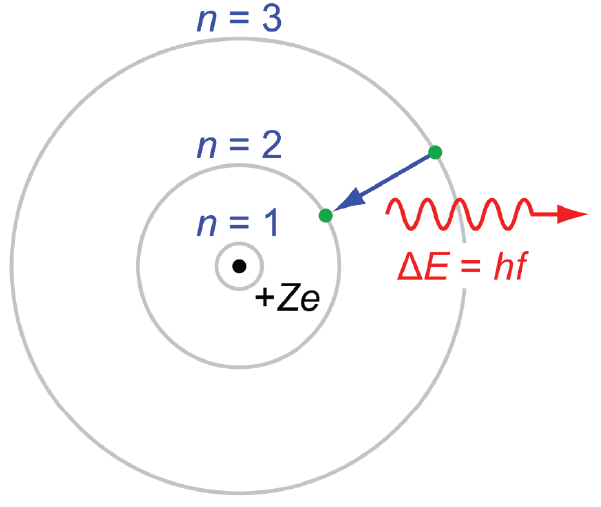
Bohr modeled a hydrogen atom with an electron able to sit in discreet energy levels, and the emission of light (the line spectrum) from the hydrogen atom comes from an electron transitioning from a higher energy level, to a lower energy level. The difference in energy between the two levels is given off as a wavelength of light. The idea of those energy levels are shown in the model above and the amount of energy can be described by the following equation:
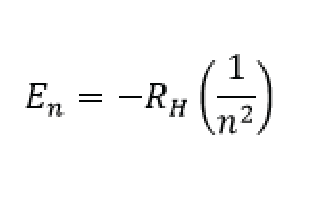
In this equation En is the energy of the electron, RH is the Rydberg constant and is equal to 2.18 X 10-18 J, and n is the integer corresponding to the energy level and is called the “principal quantum number.”
When we think about the amount of energy given off from a transition from one energy level to the next we are thinking about (delta)E = E(final) – E(initial) or the change in energy is equal to the energy of the level we are at, minus the energy of the level we were at. Since we know that Each of those energies is equal to the Rydberg constant multiplied by 1 over the square of the principal quantum number we can re write (delta)E = E(final) – E(initial) as the following:
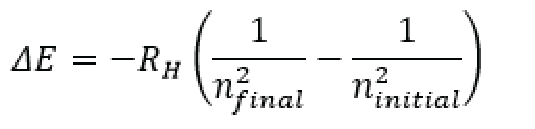
Since we also know that Energy is equal to hv (frequency * plancks constant) then we can also say that the emission of the energy from the hydrogen atom, which is equal to the transition from the energy states defined by the equation above is equal to hv.
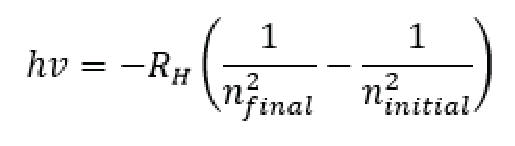
Things can get even crazier, remember in the example up top where the laser was shining in your eye we said that the frequency (v) was equal to the speed of light/ the wavelength of the light v= (3.0x108/λ) well we can replace the frequency in the equation above:
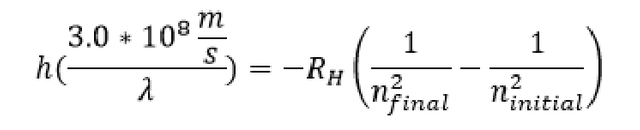
Now all of the sudden we have an equation to relate the wavelength of light given off, to energy levels of electrons that are whizzing around in a hydrogen atom. (I said there was a purpose for all of those lasers and light talk in how we built up our understanding of atoms). For now, we will stop here (as this post is getting really long!) We will pick up from here with people confusion about Bohr’s model.
I hope this post has helped you begin to get a bit more of an understanding the craziness of the work that was done in the past to help us begin to get a better understanding of what atoms were. Also keep in mind that much of what I described to you isn’t quite right, science is an iterative discovery process. New ideas are built upon the old, and as we understand things better, the old ideas get replaced. It’s still valuable to go back and build our own personal knowledge starting from the ground on up!
I also welcome all of the physicist Steemians to tell me where I am wrong in this simplistic history lesson of atoms!
Future Posts
Subsequent posts will cover: MORE Electronic Configuration of Atoms, Chemical Bonding, and Molecular Geometry, and more.
Reference Figure: Periodic Table
Other References
Constants and Conversions List
Source for Additional Constants
Some Common Ions
An Open Source Chemistry Text Book
Additional Sourcing Information
General Chemistry: Principles, Patterns, and Applications
General Chemistry: The Essential Concepts
General Chemistry
https://en.wikipedia.org/wiki/Bohr_model
https://en.wikipedia.org/wiki/Max_Planck
http://www.physlink.com/education/askexperts/ae24.cfm
If you like my work, please consider giving me a follow: @justtryme90. I am a PhD holding biochemist with a love for science. My future science blog posts will cover a range of topics in the biology/chemistry fields.
Thank you for your support of my work! I appreciate every comment (I try to make sure to respond to every one of you) and every upvote! If you would like to discuss anything from any of my posts in more detail, please join us in the steemSTEM chat on steemit.chat!
Thank you Rob, very interesting and funny post! Waiting for the next one.
Thanks for the kind words @mdcomes, I will try not to take as long as I did for this one. :)
Try these things to make formulas look cleaner in future:
They must have fixed that because as little as two weeks agodidntwork. Steemit would come up with an error and not let you post at all if those commands were included. Thanks for letting me know it works now, I will go back through and edit this post to incorporate proper subscripts! Personally I would prefer to use a star for multiplication...
No problem! Yeah, there was also a brief period when sup worked but sub didn't. 😄 I thought I was doing something wrong when sub wouldn't work. lol
Hmm. An asterisk is pretty clear, but yeah, it can come across as a formatting character in markdown editors. I guess you could always use one of these, too: ⋆, ✷, ⭑. They are actually star shapes, so the editor won't convert them.
The one star is pretty perfect for use, thanks for those!
No problem!
Very interesting - I will give these post to my daughter. She starts with chemistry this year :-)
Awesome, I am glad you think they are good! :)
hated chem in high school hehe
That just means your high school chem teacher sucked! LOL.
If I taught High School chem we would spend a good portion of the time learning about reactions and blowing shit up with them. I think I could make it pretty exciting most of the time and overall pretty enjoyable.
hehehe sounds cool
Thank you so much for posting this. I am going to go back and read the previous posts before reading this, but just scanning over it I can see that you have added incredible value to the community. This is what Steemit is all about (IMO)...
I'm having fun, I try to involve my self in lots of things that hopefully will be bring value to us all :) Teaching others is something that I feel steemit can be uniquely useful for! Thank you for your kind words, comments like this are what really makes the time I put into writing this stuff worth it!
Damned! That's not a chemistry lesson but a quantum mechanics lesson. I hope I don't have to write a chemistry lesson instead... I will not be able to do as good as you did for a physics lesson :D
(Don't worry, I still have complementary information to bring, and this could happen in 20 days.)
There are however two details I don't totally agree with you (but I partially do, don't worry, and those are details).
In fact, Planck deduced that the energy was quantized solely in the context of the black bodies. Then, Einstein reinterpreted Planck's work and generalized the concept as a property of any radiation.
The Rydberg constant value is 1.0967758 × 105 cm−1. It is not an energy.
PS: there is a planks constant on the text that should be a Planck constant ;)
We cover this stuff too in a standard freshman undergraduate general chemistry course. :) Just not in as much detail as is done in physics.
There were 2 "plank"... how embarrassing, can't even spell the man's name right. The Rh constant value I gave is 1.0967758 × 105 cm−1/hc ... but I didn't state it. ;)
I hope I didn't steal any of your upcoming post's thunder @lemouth!!
Don't worry, I have plenty of complementary material you haven't mentioned at all :)
Whoops, final and initial are flipped in my images, going back to fix that!
Edit: Fixed, sorry about that.
This post has been linked to from another place on Steem.
This post has also been linked to from Reddit.
Learn more about and upvote to support linkback bot v0.5. Flag this comment if you don't want the bot to continue posting linkbacks for your posts.
Built by @ontofractal