Photo-disintegration: Splitting atomic nuclei with light (kind of)
Today I'd like to talk about something photodisintegration: A nuclear reaction in which a very high energy photon (particle of an electromagnetic wave) splits apart an atomic nucleus, breaking it into several parts not unlike the fission reactions in nuclear reactors and weapons. Someone mentioned photofission (a similar process) to me today, leading me to remember a bunch of things about photodisintegration, so I'd like to share this topic with you, as I find it pretty cool.
This nuclear reaction was used to measure the small difference in mass between the neutron and proton, and can be used to produce free neutron radiation.
Gamma Rays and Background
When I say in the title that light can split atomic nuclei, I'm exaggerating a little bit. Ordinary visible light cannot break up any nucleus, but gamma rays (higher energy electromagnetic radiation) can, in some cases. The reason that I am referring to gamma rays as "light" is because other than the energy difference, they really can be thought of as the same thing.
Light, radio-waves, gamma rays, X-rays, infrared, UV rays, and microwaves all make up parts of the electromagnetic spectrum, and all are electromagnetic waves. Electromagnetic wave properties depend strongly on their frequency, which in turns determines the energy of an electromagnetic photon. Photons are the quantized particles of electromagnetic waves, and each photon has a certain energy. The frequency of certain electromagnetic waves determines the color of visible light, the radio channel you are listening to, and which parts of your body show up on a X-Ray image.
Photon energy determines whether electromagnetic waves can change electron energy levels, ionize atoms, break up molecules, excite nuclei, and, as is the subject of this post, indirectly destroy atomic nuclei. You can find the energy of a specific photon by multiplying its frequency by Planck's constant h. As an example, red light is made up of photons with lower energy and frequency than photons making up blue light. X-Ray photons are many times more energetic than visible light photons, which are in turn much more energetic than infrared photons and microwave photons.
Gamma rays are electromagnetic waves produced by nuclear reactions. As such, they technically don't have to be more energetic than X-rays (produced by electron interactions) but in almost all cases, gamma rays have more individual photon energy than X-rays. Typical gamma ray energies from common nuclear decays run from 10,000 electronvolts well up into the millions of electronvolts (for perspective, a visible red photon has an energy of about 1.8 electronvolts - far less!).
Nuclei produce gamma rays
Credit
Typically, gamma rays are produced by transitioning energetic nuclei. This basically means that an atomic nucleus drops from a high energy level to a low energy level, emitting a gamma ray in the process. It's somewhat difficult to find an example of this that's relatable, but a decent example is the Americium-241 in your smoke alarm. Many smoke alarms contain this heavy radioactive metal that decays via alpha decay, splitting up into the lighter Neptunium-237 and a helium nucleus. However, in a decent amount of cases the Neptunium-237 nucleus produced in the decay is not at its ground energy state: Rather, it is in an excited state. This nucleus quickly drops down to a lower energy state, emitting a gamma ray - in the case of Am-241, this is usually a 59 keV gamma ray, and this gamma ray emission is the reason that you can pretty easily detect smoke alarm source plates with geiger counters.
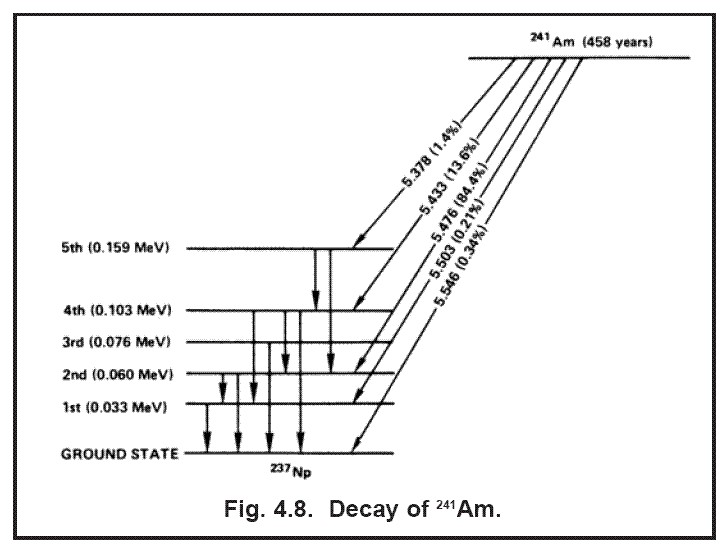
Diagram of Am-241 decay, a good example of a gamma emission event. This nuclear reaction takes place thousands of times per second in many smoke alarms.
Credit
But most reactions work in reverse. If an excited nucleus can emit a high energy gamma photon, then a high energy gamma photon can excite a nucleus to a higher energy state.
Not just any gamma ray can do this, however: It needs to have sufficient energy to excite a nucleus up to another energy level. These energy levels are separated by discrete chunks of energy.
Photodisintegration
Sometimes, a gamma ray can excite a nucleus to such a high energy that it quite literally falls apart. While this is technically two discrete processes (a gamma photon excites a nucleus, then the nucleus decays into several other particles), the end result is that what is essentially a super-fast electromagnetic wave (just like light but higher frequency) is quite literally destroying a nucleus, breaking it into smaller elements or protons/neutrons.
Photo reaction example
Credit
The textbook example is Deuterium disintegration. Deuterium is an uncommon, but naturally occurring, hydrogen isotope (one in every few thousand water molecules has a deuterium atom instead of a hydrogen atom). Ordinary hydrogen ("protium") has a nucleus consisting of nothing but a single proton. Deuterium on the other hand has a nucleus with one proton and one neutron. Unlike tritium (the next hydrogen isotope), deuterium is totally stable and not radioactive.
However, fire a gamma ray with an energy of 2.22 MeV or more at a deuterium nucleus and the nucleus can be ripped into its constituent parts: A neutron and a proton.
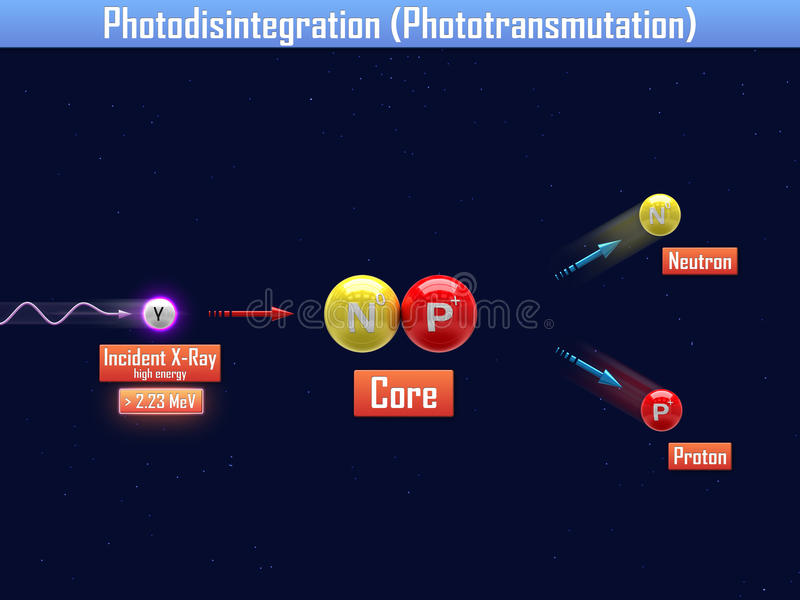
Despite the stock photo watermark I quite like this diagram of the process.
Credit
However, a neutron-proton pair weighs more than a deuterium nucleus (deuteron). This means that the products of the nuclear reaction have more mass than the input - how can this be? To rectify this, remember that nuclear reactions don't conserve mass: They conserve energy, which is really the same thing (see Einstein's equation). No energy was lost here: The extra mass came from the 2.22 MeV of energy in the gamma photon.
High energy photons have some other interesting possible interactions that superficially seem to "create mass". For example, if a gamma photon has an energy of 1022 keV or higher, it can interact with a heavy nucleus and produce a positron-electron pair in a process known as pair production. Since mass and energy are for our purposes equivalent, this massless photon produced two massive particles from seemingly nothing. Pair-production is one of the ways to actually make electrons, with their own 511 keV mass. Remember, this "extra mass" simply came from the gamma ray - no mass was actually created. The same occurs in the photodisintegration of deuterium.
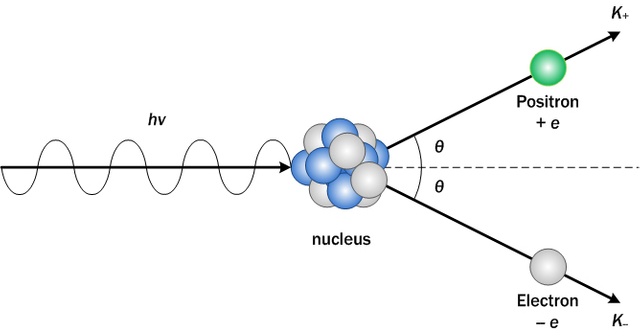
This is a pair-production reaction, which also produces more rest mass than it started with, with the extra mass coming from the gamma ray energy.
Credit
Essentially any atomic nucleus can be destroyed via gamma ray disintegration - you just need to use a gamma ray of sufficient energy. The lowest energy photodisintegration reaction I've been able to find is the photodisintegration of Beryllium-9, the stable yet toxic beryllium metal. This reaction requires a 1.67 MeV or higher gamma ray, which is low enough that several nuclear decay reactions can produce this kind of energetic gamma ray. The Be-9 gamma ray reaction sees the gamma ray break the Beryllium nucleus into two Helium nuclei and one free neutron (remember, there are actually two processes here: Gamma ray absorbed, and nucleus decays).
Interestingly, for heavier atoms (past Iron on the periodic table) photodisintegration can actually release energy - that is, you will produce more kinetic energy than you put in, since the decay products will weigh less than the original nucleus. For the deuterium splitting, the reaction consumed net kinetic energy, making it endothermic.
Photodisintegration Uses
There aren't a huge amount of practical uses for these kinds of gamma ray induced reactions that I know of outside of scientific research. However, one practical use is as a neutron source.
Neutron sources are used for a ton of things: They can be used to identify what elements are in any material, create images of shielded containers, initiate nuclear reactions to study, or kick-start nuclear reactors. I wrote a short article on how a simple one is made here if you're interested. Photodisintegration neutron sources work by mixing an element that will split into neutrons and other nuclei upon contact with gamma rays with a gamma-ray producing radioactive element. An example I was able to find online was mixing Antimony-124 with Beryllium-9, which will form a radiation source that emits free neutron radiation in all directions via a photodisintegration reaction.
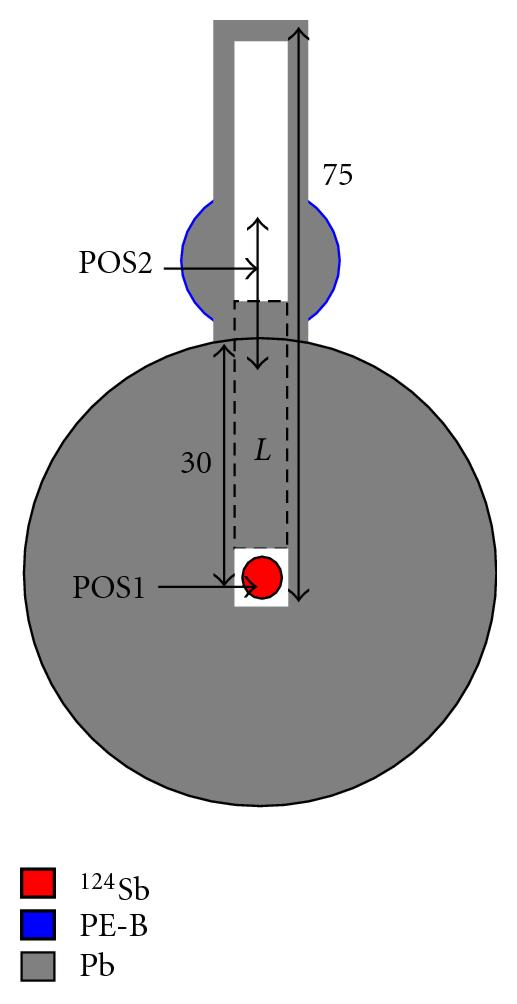
I found this diagram of a Sb-Be neutron source, to be used in a reactor.
Credit
Of course, scientific nuclear physics research has many additional uses for these reactions. One such use is to measure the mass difference between the proton and neutron. This was what was done by the first photo-disintegration experiment that I know of (from Chadwick and Goldhaber), and used a Thorium decay product (referred to them as "Thorium-C''") to split deuterium nuclei.
Hopefully you were able to learn something new about photo nuclear reactions today! I found these to be particularly cool because they are initiated by what is essentially super-high-energy light (even if it really acts nothing like light). Unfortunately I haven't found a way to detect these reactions in a DIY setting so I won't be to try this myself.
Let me know if I got anything wrong, or if you have any questions, comments, or criticism.
Thanks for reading!
Sources Used for this post:
A nuclear 'photo-effect': disintegration of the diplon by γ rays | Chadwick and Goldhaber
On the photodisintegration of Beryllium and Deuterium | Myers and Van Atta
Photodisintegration Wikipedia Entry
Am-241 Decay Factsheet
Startup Neutron Source Wikipedia Entry (for Sb-Be Neutron Source)
Thanks for posting. I've now a backlog of Steemit posts to read, this being one of them. Photodisintegration occurs also in supernova of ultra large stars. Have so much reading to do :)
You do a great job with damping down on the jargon in these posts. I'd love to hear your take on Low Energy Nuclear Reactions.
https://edgylabs.com/lenr-cold-fusion
http://ecatworld.org/what-is-the-e-cat/
https://www.scientificamerican.com/article/cold-fusion-lives-experiments-create-energy-when-none-should-exist1/
Thanks! I'm glad the post was at least somewhat easy to read.
Interesting articles, in particular the last one. One of my biggest issues with cold fusion/lenr claims is that they never release radiation. Essentially all nuclear reactions produce large amounts of high energy ionizing radiation - after all, that's why nuclear reactions produce a lot of energy in the first place. Because of this, a claim that fusion is occurring in an electrolysis device for example seems strange because the device isn't setting off every geiger counter in the room. For example, D-D fusion (the second easiest fusion reaction) produces tons of neutron/proton radiation and some gamma radiation. Significant enough amounts of D-D fusion to heat water would produce so many neutrons that even the simplest radiation detectors could pick them up. I'm of course aware that not all lenr claims involve D-D fusion, but essentially any nuclear reaction produces high energy particles. In addition, if there actually was something going on, it should be simple to provide a demonstration of more energy out than in.
So I'm quite skeptical of these claims. The E-cat in particular seems very suspicious. That being said, we obviously haven't discovered everything about nuclear reactions, so there could definitely be a way to produce nuclear fusion in a simpler way than large plasma colliders - it's just that any such fusion event would be easily detected by the vast amounts of radiation produced. It's also important that claims be checked out to see if they do hold any water. If there is ever conclusive proof of a new way to induce nuclear reactions, I'll be the first to recognize it.
Changing a massive part of what we know about physics (such as Mills is trying to do in the last article) requires extraordinary proof.
Thanks for linking these articles, interesting reads.
See, that's what I'm talking about. "Look for neutrons." A simple answer that makes sense. Where might the "extra" heat be coming from?
I have another physics paper I want to ask about, but I'll have to find it again.