The Ridiculously Simple DIY Neutron Radiation Source - Helium Beryllium fusion
Hello everyone,
This post is about a very, very, very easy to make source of neutron radiation. So easy that assembly literally involves taping two things together. It also happens to be essentially the only source of neutron radiation that you make without building a very expensive difficult-to-make fusor or breaking the law. The catch? It's not really useful. But I think it's really cool to discuss. Let's get started!
Quick safety notes before I begin:
- This involves two different extremely toxic elements
- It involves multiple types of ionizing radiation
- It may not be legal in your country
- It doesn't produce enough of, well, anything to be that useful for experiments
So I really shouldn't have to say it twice: Don't do this. This post is for informational and educational purposes only, because I think it's really cool that this is possible. I haven't personally done it because of the above reasons. However, by reading this you might become a little more educated about nuclear reactions, which is the goal here.
Neutron Radiation
My last post detailed free neutron radiation in more detail (Click here if you'd like to read it). The short of it is that free neutron radiation is relatively rare on Earth and has some interesting properties. Neutrons are somewhat tough to shield but certain materials will absorb them quite well if you are able to slow down the neutrons. In addition, neutrons can transmute nuclei into other isotopes, which will then usually decay into a different element. In effect, neutrons literally allow you to do alchemy (there is a way to turn other elements into gold using neutrons), although on scales so small you wouldn't even know without a gamma spectrometer. Neutrons are cool, and also dangerous in large amounts.
Unfortunately or perhaps fortunately, neutrons are not at all easy for a hobbyist to make. Your options include building a fusion reactor and fusing deuterum to produce proton and neutron radiation, which is actually possible, but will cost you a lot and will take a really long time (it's something I want to eventually do if I ever have more money and time. Look up "DIY Fusor" online to see what some incredible people have done with these machines). Or, there is the Alpha-Beryllium neutron source, which is what this post is about.
Alpha Beryllium Neutron Source
All nuclei are positively charged once you strip away an atom's electrons. This means that two nuclei will repel each other. If you get them closer together, they repel even more. If you get them really close together, however, there is a chance that a quantum tunneling event takes place and the nuclei get close enough together for the strong force to take hold, overwhelming the electromagnetic force and fusing the two nuclei into one big nucleus that will almost certainly decay and break up shortly after. This is how nuclear fusion works, and quantum tunneling is the only reason that the sun is able to fuse substantial amounts of hydrogen (without it, you would have to completely overcome the electromagnetic attraction to fuse nuclei, which would require much higher kinetic energies and temperatures).
Alpha radiation is typically in the range of 5 MeV per helium-4 nucleus. When this radiation strikes a beryllium target, some of the alpha particles that hit beryllium nuclei head-on will fuse with the beryllium nucleus due to their high relative kinetic energy. Now technically this is called an alpha transfer reaction, but it's basically fusion since two elements fuse to make a third different element, so I'm called it fusion unless someone corrects me since it sounds better. Just think of it as fusion with the particle accelerator being a heavy decaying nucleus.
The most common reaction when a Helium-4 alpha particle fuses with a Beryllium-9 nucleus produces a Carbon-12 nucleus, a gamma ray, and a free neutron. Since the alpha particles are "pre-accelerated" for us up to about 5 MeV (which would be very difficult to do artificially with an electrostatic accelerator, as you would need a 5 MEGAVOLT supply!), this reaction is very easy to induce, provided you have an alpha emitter.
The relevant reaction is nicely formatted in the following image, thanks to Wikipedia:
But where to get the alpha radiation for this reaction?
Alpha Emitters for the Hobbyist
Alpha decay is actually pretty common as far as radioisotope decays go, and it occurs mostly in very heavy elements. Now, you could go off to a professional supplier website and buy legal sources of alpha radiation. But this would be very expensive. Or, you could go on Ebay and buy some Uranium ore Geiger counter test samples, which also produces lots of alpha radiation. But this alpha radiation is dispersed over the surface of the rock and not very concentrated, and besides, who really wants to deal with an ore that could produce dust that you could breath in?
The easiest alpha source is Americium-241, because it's found in many smoke detectors. These smoke detectors can be purchased for a few dollars online. Inside is an exempt source, 37 kBq (37,000 decays per second) of Am-241. Am-241 has 241 nucleons (neutrons plus protons) and occurs on the periodic table at element #95, past Uranium. It decays into Neptunium-237 with a half-life of about 400 years. Each decay produces a 59.5 keV gamma ray (easily shielded by some metal foil) and a 5.5 MeV alpha particle. Remember, alpha particles are just high speed Helium-4 nuclei. The result is an alpha source that's actually pretty safe, and legally sold in the United States (the gammas are easily shielded, the alpha radiation is incredibly easily shielded, and the source isn't very active anyway at 37 kBq so there really isn't a health risk ... UNLESS YOU EAT IT).
Now I don't actually recommend you take these apart unless you know what you are doing, because airborne Americium-241 is toxic in so many ways even in small quantities. That being said, if you were to take apart an ionization smoke alarm you would find this:
The little gold circle is the Am-241 source (the americium is often bound in a gold or silver matrix to prevent it from flaking off into the air).
Now, all that remains to making a pathetically low output neutron source is taping the alpha emitter to a block of beryllium.
By the way, beryllium-9 is extremely toxic even if it isn't radioactive. If you breath in too much of it you can get berylliosis, a PERMANENT lung disease. Don't mess with this stuff and certainly don't grind it up. As I said before, there's no real reason to recreate this and the whole point of this post is to demonstrate some cool nuclear physics/reactions, so please don't go buying beryllium or dissecting smoke alarms. The beryllium, by the way, is definitely the more hazardous of the two.
The Neutron Source
Great, so hypothetically we have our beryllium chunk and alpha emitter. All there is to making the (say this in a dramatic voice) Alpha Beryllium Fusion Neutron Source is taping the beryllium block to americium source. That's it, it's literally two metals taped together. The least complicated device ever. But it does work. Let's see how well (or poorly) it performs:
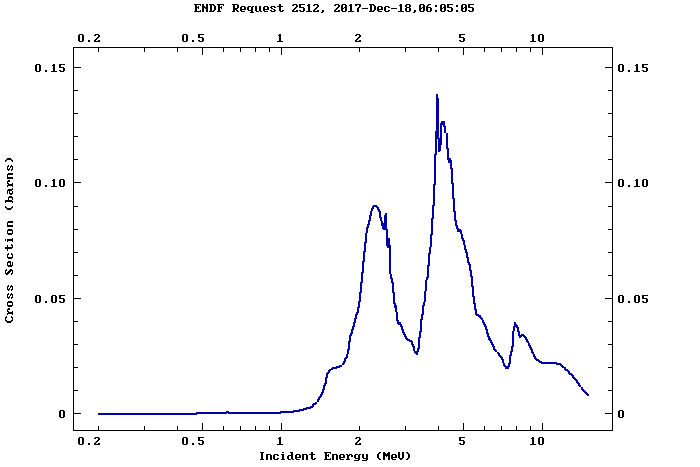
While you could go out calculating cross-sections and reaction probabilities based on each alpha particle, this is annoying and slow (although I encourage you to try it if you are curious: https://www-nds.iaea.org/exfor/endf.htm has all of the reaction cross sections you could ever dream of). Wikipedia provides us with an estimate: "30 neutrons for every 1,000,000 alpha particles". Having seen several successful alpha beryllium sources online, I'm inclined to believe them.
By the way, here's a nice plot of the reaction cross-section from the ENDF database linked above. Notice the peak right around 5 MeV.
So, let's assume that half of our alpha particles in the 37 kBq source leave the source (the other half, being fired into the metal, don't get far). That leaves us with 18,500 alpha particles per second leaving the front of the source. 30/1000000 = 3e-5 is the fraction of alpha particles striking the beryllium that will induce a fusion reaction and produce neutrons, under an absolute best case scenario. Since the americium isn't physically inside the beryllium, let's throw in another factor of 1/2 for accuracy - that leaves us with a reaction fraction of 6e-5. Finally, multiplying the number of incident alpha particles per second by the reaction fraction gives us ... a whopping 1.1 neutrons per seconds (round to 1 because it's a nice number).
And remember, this neutron can be emitted from any side since it's a neutron that will most likely ignore the metal surrounding its creation points. And that is why this is basically not a useful device in the small, legal quantities we can make: You'd have trouble picking up 1 neutron per second with good equipment, let alone the scrap-bin detectors I'd be able to come up with. What's more, neutron detecting equipment is fundamentally more expensive than other ionizing radiation detecting equipment unless the neutron flux is massive (hint: 1 neutron per second is not massive).
Basically: You'd have trouble picking up this 1 n/s over the background neutron flux from cosmic rays.
But: If you stretch it just a little bit, it's technically a mini fusion reactor in your pocket (don't actually put this in your pocket, that would be somewhat unhealthy). That's a little clickbait-y, but it's true: The two metals taped together is actually starting reactions that fuse two different elements into a heavier element and output gamma rays and neutrons. Even if the only effort you had to expend to make it was to tape two things together (after obtaining the alpha emitter of course)
And that's it for the only neutron source you can make for $10. Once again: Don't make this, it's somewhat of a health hazard if you don't know what you're doing, and I think I've conclusively demonstrated that it's not useful for experiments.
Could you transmute elements with this? Well, yes, but you'd never be able to detect it and you'd be making less than 1 atom per second which is essentially nothing. Again, background neutrons would probably beat you if you tried to actually use this source for a science experiment.
I hope you learned something new about nuclear reactions from this post. If you have a question, shoot a comment below and I'll do my best to answer. Remember to be safe and don't replicate this, berylliosis is no joke.
Thanks for reading!
Tips/Upvotes appreciated!
NAV: NZLuonFWZTRL8mYQ41SwaP7qopSTbgDQ72
BTC: 1MV7pvR9PGzYBMbSpAyRQbMBonJxZyBnrT
VTC: ViuLmSCuyQXLTJKZphKPwmTvFfvjc88Woz
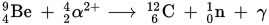

Congratulations @proteus-h, this post is the tenth most rewarded post (based on pending payouts) in the last 12 hours written by a Newbie account holder (accounts that hold between 0.01 and 0.1 Mega Vests). The total number of posts by newbie account holders during this period was 2650 and the total pending payments to posts in this category was $1012.53. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
Sorry friend. My English not very good. But I read whole post. I think I understand 100%, but, to confirm: we buy smoke detector, we take apart, we grind gold circle, we take beryllium, we smell deeply, we connect with Americanum US 241, and put in pocket, because berylliosis is healthy for lungs. Correct?
Can I mine bitcoin with this?
You have been mentioned!
good post. mi like the science
Congratulations! This post was randomly selected and upvoted by @resteemr!
@resteemr is curating posts tagged #technology this week.
Read More about it here.
Is it dangerous? Haha. No, for real, I'm a little bit scary to di this at my home.