The neutron darkness in a bottle (and a beam)
All matter around us is constituted of particles that are stable. This is a very good thing. It means that the constituents of our world are stable and will not decay. We indeed do not want to see our favorite cup of coffee decaying into something else before having the time to drink it…
[image credits: Pixabay]
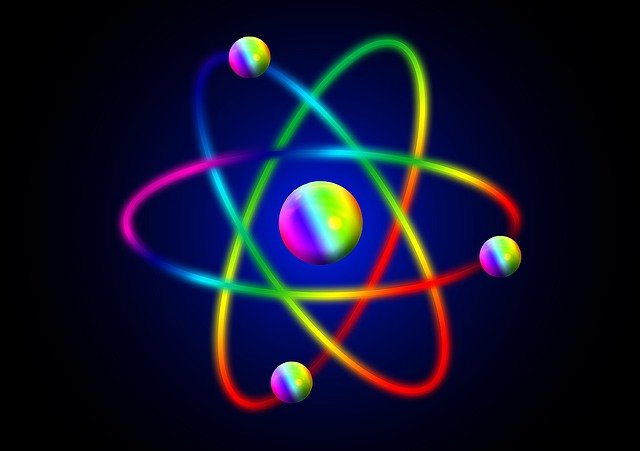
For once, I will not discuss elementary particles, but restrict myself to a larger scale. I hence start my discussion at the level of atoms.
To summarize what can be observed in the picture on the left, atoms are made of an atomic nucleus and of electrons that orbit around the nucleus.
We however know for more than a century that the atomic nucleus is a composite object whose building blocks are protons and neutrons.
Here are thus our stable particles: protons, neutrons and electrons. To this list, we can add neutrinos (that appear in decay processes of an atomic species into another) and photons (that are connected to the electromagnetic interactions of our atoms).
In this post, I will focus on the neutron. Although I have stated that the neutron is stable, it actually decays after 15 minutes (wow, a first contradiction?!). Moreover, the measurements of its lifetime exhibit a puzzle that lasts for more than 20 years (a second contradiction!).
This puzzle attracted a lot of interest recently, as it could potentially be solved by dark matter, although neutron stars made this challenging.
WHY HAVE I SAID THAT THE NEUTRON IS STABLE?
I started this post with a pair of contradictions. Let us focus on the first of these.
Within a couple of sentences, I have mentioned that neutrons can be classified as stable particles, and also that they decay in about 15 minutes.
[image credits: Wikimedia]
The second part of the above statement may seem frightening: neutrons decay in 15 minutes. Knowing that neutrons are everywhere in normal matter, this may indeed be worrisome.
But there is actually no reason to panic…
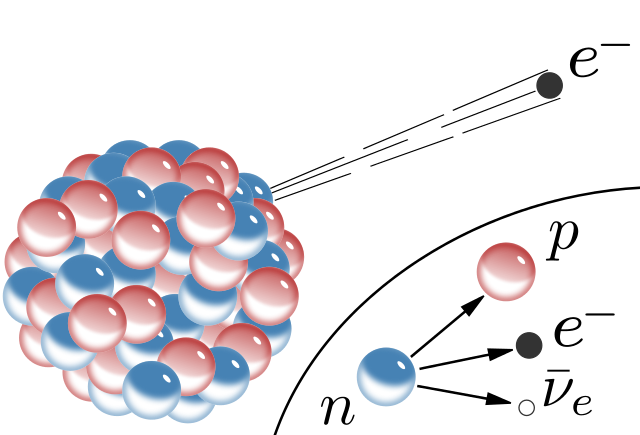
It is true that neutrons are unstable. One can indeed take a neutron, wait for about 15 minutes, and one will get a proton, an electron and a neutrino.
No more neutron! The beta decay mechanism did the rest (as sketched on the picture).
What saves matter is that within an atomic core, neutrons are not alone. They are surrounded by other protons and neutrons, which collectively makes the atomic nucleus a stable system.
I hope now that this contradiction about the stability of the neutron is settled. Let us thus go back to the topic: neutrons are unstable.
Consequently, experiments have been conducted for decades in order to try to measure the neutron lifetime. This is after all one of the crucial properties of the neutron and is thus need to be measured.
We have two independent classes of experiments here, using respectively the bottle and beam methods.
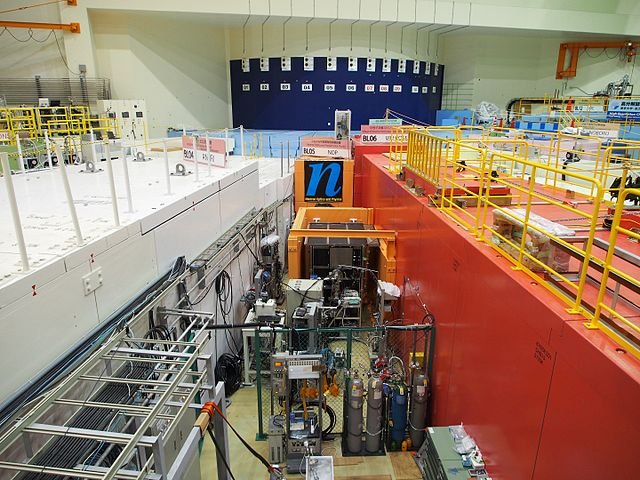
NEUTRONS IN A BOTTLE
[image credits: Wikimedia]
One of the two classes of experiments aiming to measure the lifetime of the neutrons is very simple. It is called the bottle method, and for a very good reason.
First, we collect a bunch of neutrons and store them in a bottle. In fact, we actually trap the neutrons, by means of a combination of magnetic fields or material walls for instance, so that we can count them.
We have thus a kind of bottle with neutrons in it, and we know exactly how many neutrons we have at the beginning of the experiment.
Then, we wait… wait… and wait a bit more.
After some time, we check how many neutrons remain in the trap. From that number and the initial number of neutrons, we can evaluate the decay rate of the neutrons, and thus the neutron lifetime.
This is what has been done in particular at the Laue-Langevin Institute in Grenoble. Ultra-cold neutrons have been trapped, as detailed in this open-access publication. Thanks to a bunch of neutron counters, it was possible to follow the evolution of the number of neutrons with time.
The measured neutron lifetime has been found to be 879.6 ± 0.6 seconds. Roughly 14 minutes and a half. And it can be seen that the measure is damned precise.
NEUTRON BEAMS

[image credits: Wikimedia]
The second class of experiments targeting the measurement of the neutron lifetime relies on neutron beams.
For a very inventive reason, the method is known as the neutron beam method. Yeah, physicists are good with names ;)
Here, we prepare a beam of neutrons (in case you did not guess from the name of the method) and we measure the amount of neutrons decaying over time in a given volume of the beam.
This number is derived by counting the rate of protons that are emitted by the decaying neutrons. This in fact measures the beta-decay-rate of the neutron, as each neutron is assumed to decay into a single proton, an invisible neutrino and an electron.
The most precise measurement of the neutron lifetime within this method can be found in this publication, and it is found to be 888.0 ± 2.0 seconds.
This number is very close to the previous one, but different enough!
DISCUSSION: A DISCREPANCY SOLVED BY DARK MATTER?
As I have detailed in this post, one can use two independent methods to extract the neutron lifetime (the so-called bottle and beam methods).
Until now, both methods have been found to disagree with a difference of about 8.5 seconds. Even after accounting for the size of the error bars, the two measurements still disagree. We are talking of a disagreement of more than 4 standard deviations (i.e. a disagreement at the 99.993666% level).

[image credits: Wikipedia]
One possible catch is that with the neutron beam method, one only focuses on neutron decays into protons. In contrast, with the neutron bottle method we focus on all neutron decays.
‘All’ is the important word. This means, also non-standard decays that no one is expecting and that could not be observed.
Physicists have tried to investigate this path, and have proposed models where neutrons could decay into a very light dark matter particle, as shown for instance in this work.
The interesting fact is that even if this scenario is challenged experimentally, it is still viable. In particular, neutron stars can be problematic, as I have written in this old post.
Therefore, there is only one wait to know more about this puzzle: wait for more data.
SteemSTEM is a community-driven project that now runs on Steem for more than 1.5 year. We seek to build a community of science lovers and to make the Steem blockchain a better place for Science Technology Engineering and Mathematics (STEM).
More information can be found on the @steemstem blog, on our discord server and in our last project report. Please also have a look on this post for what concerns the building of our community.
So, as of now it is unclear that what happens to these decayed neutrons. Isn't it? Also I want to know whether the existence of Dark Matter still a theoretical concept?
Thanks for your message. There are several questions in there. Let's first answer the first one:
This is indeed a hot topic of research. We don't know with enough certainty what could be the cause of the difference between the two measurements, and we are trying to explore all options. The dark neutron decay is one of them.
There is a lot of experimental evidence pointing to the existence of dark matter. However, for some reasons, dark matter still evades direct detection and we have thus very little information on its nature. As above, we are trying to explore all options.
Note that theories without dark matter, in which gravity is modified, are also viable alternative. But they are not as strongly supported by data (even if not excluded).
Thank you @lemouth
You are welcome :)
So if you have a bunch of neutrons in a bottle, why can't they form a nucleus and then an atom. When some of them decay to protons and electrons you have all required parts.
The nuclear force binding an atom has a very short distance. So, I guess the question is how close can a neutron get to another neutron?
This is an excellent question!
Electron capture by a proton requires some energy to be converted into a neutron and a neutrino. We don't have this energy available here so that the inverse reaction is not possible.
Note that sometimes, it is possible that a neutron directly decays into an hydrogen atom. This can be observed.
It is a combination of the uncertainty principle (that implies that the neutrons have some momentum when located in a zone of 1fm) and the range and strength of the nuclear force. Two neutrons (or two protons) cannot form and bound state as the above-mentioned small amount of momentum cannot be compensated by the strong force.
Does it help (I am a bit out of my field with your questions ;) )?
It does, thank you :)
You are welcome! :)
You are assuming it will decay into something worse... but maybe it will decay into a cup of artisanal home-brewed coffee made from whole beans that impart a flavor whose profile can be described as half raspberry half blow-your-mind .. with cream 🤔
That's more than twice as long as some other particles last.
This was a more approachable post than usual!
Thanks for your comment! ^^
Coffee decaying into something with cream? Definitely a decay and not an improvement! :D
Mmmhhh.... This is heavy (and hard) stuff. How do you manage to find this? :D
That particular particle I feel very connected to, so I try to learn about it! :P
And you learn twice as fast (maybe too fast?) :D
That particular particle I feel very connected to, so I try to learn about it! :P
And you learn twice as fast :D
I'm guessing if a way was found to count not only the protons but also the rest of the particles emitted, it would clearly show whether non-standard decays involve decaying into something completely different.
Unless the 8.5 sec difference can be attributed to some kind of delay during the decay process. Some intermediate state perhaps? Just a thought.
Except of the rest is not standard and if we have no idea about how to count it (because we don't know what it is).
I think that this has been double-checked (across several independent experiments of both types), and we are fully positive about a real discrepancy.
Doesn't time slow down for objects as they move closer to the speed of light? Could the fact that the neutrons are being put into a beam (in motion) account for the observed longer decay period? To the neutron, it would be decaying in normal time, at a normal rate To the observer of the neutron it would appear to be decaying slower... Maybe
Yes, your statement is correct and your guess is a very nice attempt. However, it does not apply here as our neutrons are not accelerated to speeds close to the speed of light at all. :)
It's difficult to recognize you with the new avatar...
Anyway, I remember the little hype in 2014 about the "solar influence on the decay rate"
Was there any progress in explanation of this phenomenon since then?
I was not aware of the paper by Jenkins et al. I quickly read it and it seems pretty clear that there is no connection between this and the neutron lifetime.
I have double checked and it seems that they are evidence against the solar influence on the decay rates. See here for instance. It thus looks like this puzzle is today solved. ^^
It's nice to see that it's closed topic now. The only problem is Google... When you type in the "search" field: radioactive rate constants wrong, the first two hits are from the time of hype :(
As always... the trending page :D
SteemKahn academy , wow

for what little my vote and resteem is worth these days you can certainly have it. Topnotch information, thank you
signature obligatoire :
viewed, voted, commented, and re-steemed ...
i am not a bot and i only have one account ... and im not half as bad a lunatic as it might appear but i need to hide from the normals or they come to pick my brains
anyone follows me i'll follow back within a few days
if you have complaints please check the link(where did that link go), or else just do that content dictator thing you do i no longer care ... a resteem is a resteem, atm to 365 people, thats 365 chances to have it re-resteemed,

Free speech is either absolutely free or it is not at all. It was meant to be free for everybody : how can anyone grow up in a nanny state?
maybe you can help me out .. i personally always understood that einstein never claimed you can not go faster than light with anything but a massless photon (well that would be the actual speed of light) but in fact all you would need is an infinite amount of energy to propel anything that hass mass to lightspeed but everywhere i read "its not possible" ... despite the fact that this relates to observation ... if you're reading this, any thoughts ?
Also, i have been looking for years on a gedanken related to sustainability (i can grasp concepts easily sometimes but im not quite the numbers guy : IF it comes to that point, the actual point of no return. How many humans on average would it take for all molecules / atoms on this planet to be turned into sapients due to incessant breeding and overpopulation. Since its a limited number at some point everything will be used up (i do understand this would mean the death of all organic life way before that but its a thought more like, one i can not calculate myself) .... anyone ? (if anyone still reads this this far below)
i'll try to keep an eye out ... an interface where you can sort people in tabs by subject or interest would be nice too, sorting in circles or venn-diagrams even better, alas at the moment i could barely code a hello world so im afraid i cant get to that either
that said, i said a lot again apparently ...
thanks :)
Thanks for your pretty longish comment. Let me try to answer your questions, or at least try to give some hints about them.
Special relativity tells us that nothing can go faster than light. Nothing. End of the story. Massless particles are those traveling exactly at the speed of light, and the massive ones are slower. You can accelerate (i.e. by providing energy) a massive body as much as you can, and you will get closer and closer to the speed of light without reaching it. Like 99.999999999999999% of the speed of light.
Well, this I don't know. It depends on the yearly resources of our planet I guess, which is an information I don't have.
You can start joining us (steemstem) on discord and chat with us. Maybe will we be able to help you finding what you are looking for :)
Once again, thanks for your comment! :)
thank you, maybe its time i looked into discord after all :)
it is never too late :)
considering entropy in systems with the arrow of time strictly pointing forward i'd say that could be open to debate but i doubt i could hold my own against an actual particle physicist ... its a wonderful world, like the new magic of the teknomage lol, it takes quite some imagination to get around the end of 1 and 0 to the potentially everything between for one thing and other than that im not really the numbers guy ... too much chaos inhere, i hope you don't mind if i fire some questions your way sometime, ive been looking for someone well versed in the subject with an interest and patience to empower the layman...i'll try not to overdo it but when it starts nagging at my head its sometimes hard until i get some general idea .... (im probably less weird than i sound and certainly behave better than an unobserved particle ... in most cases)
thank you for the actual interaction so far
Don't hesitate to ask questions. Never. I don't say I will answer immediately (depends on the question) but I will always try to say something, and maybe discuss the question later in a dedicated post if necessary (this happened already 4-5 times since I am on Steem).
oh i wont , straightforward is sometimes a problem for me, lol, seeing as i dont actually have any paper degree some people think that means i should shut up but i'm not really in the business of shutting up hahah. It's refreshing to find someone who actually knows their stuff AND is willing to spare time for mere mortals
The fact of not having any degree is a good reason for not shutting up and speaking! In fact, no one should never restrain himself/herself from asking a question.
At least, I am always available (and if not, I find a suitable moment later). And I actually a bunch of people who are too. But this is country and mentality dependent of course.
This took me back to my ‘Nuclear Physics’ classes which was one of the most difficult classes to pass 😊. But nowadays I think I miss those times a little. Thank you for this nostalgic moment you gave to me and thanks for this informative post.
I hope you will survive the nostalgia. Nuclear physics reminds me the very first moment of my PhD time, when I was taking care of lab classes :)
Wow, what an impressive and informative post! Especially because of the mentioned 4 standard deviations disagreement! This points out the real struggle of the discrepancy as the results appear to be very close without the measurement error.
Yes, but the error is part of the measurement. Always. In the same way, an error is always part of a theory prediction.
This means that neutrons still go through decay, even within an atomic core, or interaction with other particles prevents this? Or some sort of equilibrium is achieved as a consequence of interactions between atomic nucleus constituents?
The bound state (i.e. the atomic nucleus) will be stable by virtue of the strong interaction, regardless of the fact that some of its constituents are unstable when taken as such.