Chemistry and Metabolism of Biomolecules #5: Chemistry of Amino Acids
Amino acids, the building block of proteins. Peeping through a hole, amino acids are the end products of the central dogma.
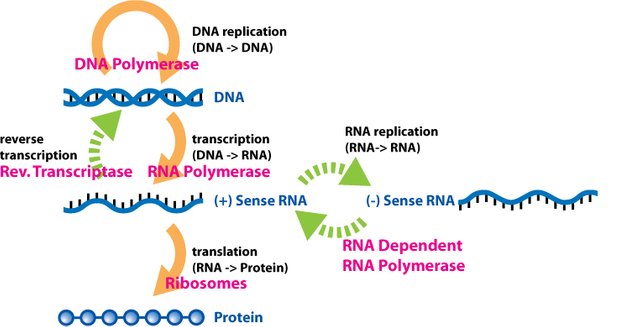
The central dogma of molecular biology shows how DNA can act as a starter molecule for the synthesis of proteins. An RNA is synthesized based on a DNA template in the nucleus of a cell, a process known as transcription
This newly synthesized RNA which is an mRNA (messenger RNA) diffuses through the nuclear membrane and makes its way to the cytoplasm where organelles like the endoplasmic recticulum and ribosomes can orchestrate it’s conversion into proteins, a process known as translation.
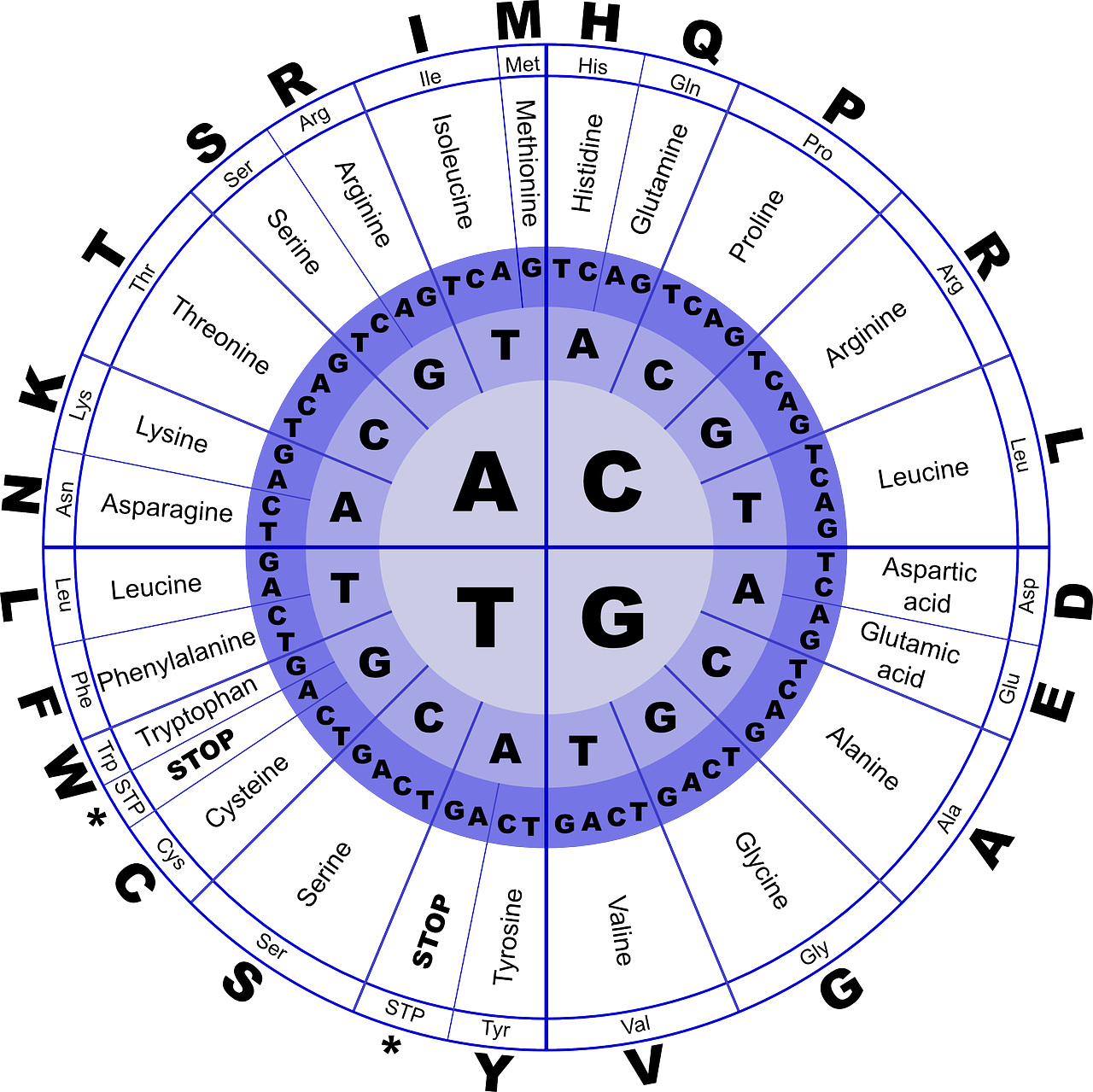
Each amino acid is represented by specific triplets of nucleotides called codon(s). More than one codon could code for a single amino acid in most cases and this is the degenerate nature of the genetic code
The aim of this installment isn’t to show you how amino acids could be synthesized however. It’s just to give you knowledge about the structural features of the existing amino acids. To begin properly;
What are Amino Acids ?
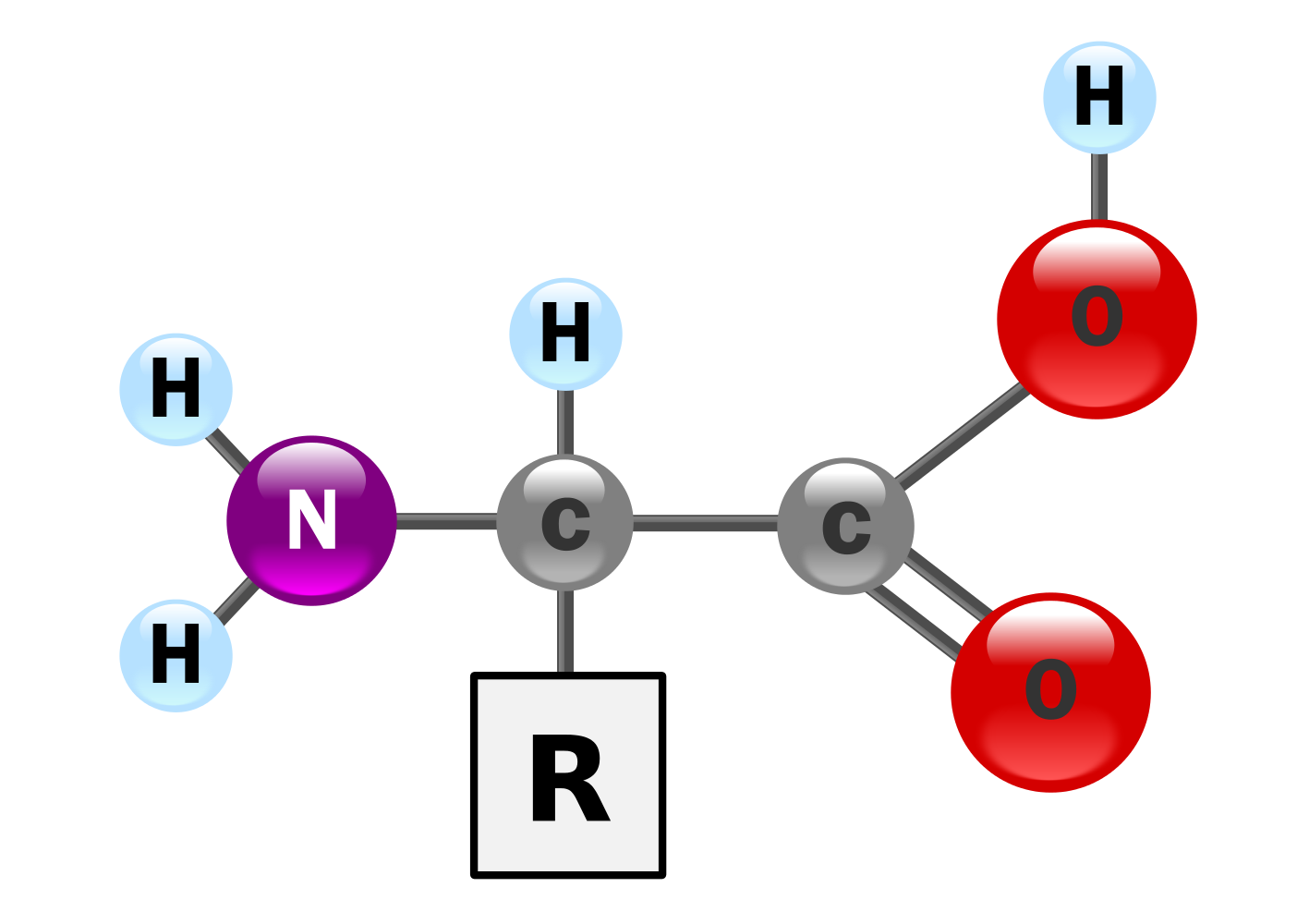
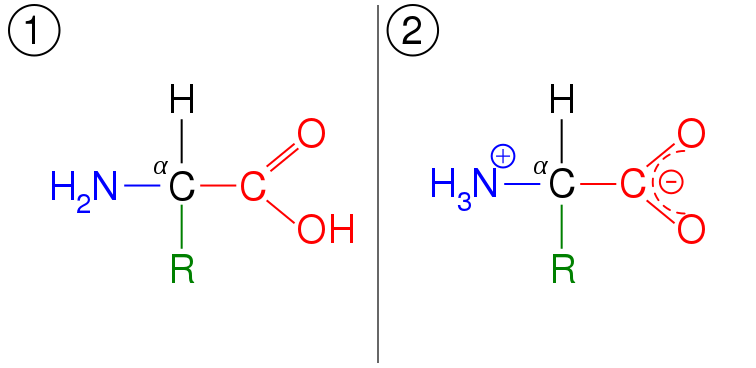
In the simplest terms, amino acids are a class of biomolecules which contain an amino (~NH2) group, a carboxyl (~COOH) group which determine its function and thus they are known as functional groups and a side chain usually denoted by “R”.
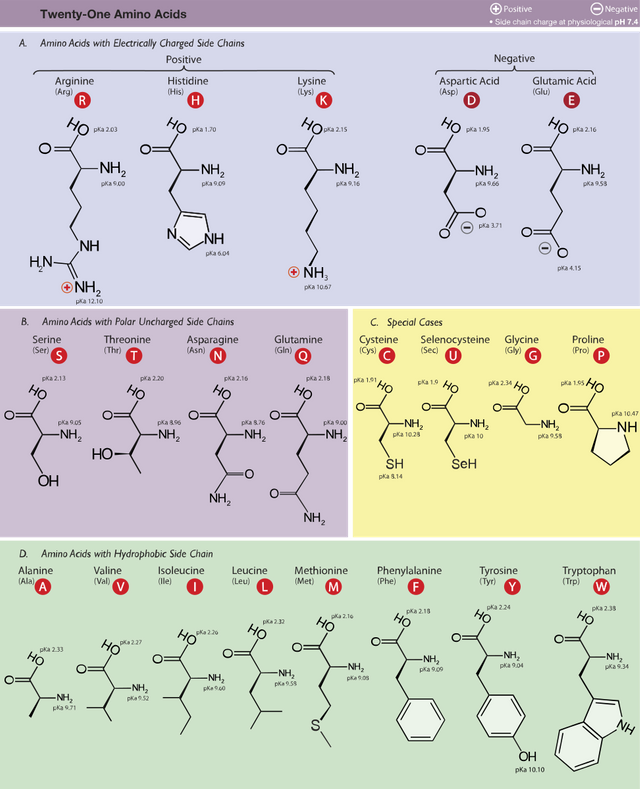
It was generally believed that the amino acids were 20 in number but that has been proven wrong with the addition of two new amino acids, selenocysteine and pyrrolysine.
The amino acids which are most abundant in nature are generally the alpha amino acids and these are amino acids that have the amino group attached to the alpha carbon. The alpha carbon is the carbon just after the carboxyl group and this can be observed by just looking at the structure of any amino acid.
Proline has shown a deviation from this regular structure of an amino acid as it contains an imino acid in place of the expected amino group.
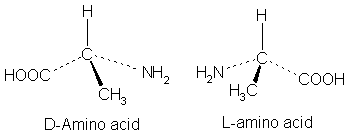
Amino acids can also show isomerism as described in one of the previous installment of this series. If you’ve been following, you can recall I said that monosaccharides exist in the D-form but the opposite is the case for amino acids. They exist in the L-form. Another exception to this is glycine.
This is majorly due to the fact that glycine contains two exact hydrogen atoms and thus does not show chirality. Chirality is only exhibited when a carbon atom is bound by four different functional groups and thus, confers on a compound the ability to exist as enantiomers
Amino acids can exhibit amphoterism. An amino acid can act as a base and an acid depending it’s oxidation state which is a function of the pH of the medium it is found. A more biochemical term to illustrate this is zwitterions.
It has been observed that the carboxyl group and amino group of an amino acid can act as a weak acid and weak base respectively. If you are a little conversant with chemistry, you’ll know that acids tend to give up their protons (deprotonation) easily while bases tend to accept protons (protonation) easily.
At certain pH levels, amino acids can accept or lose protons. At a pH < 2.2 (which is highly acidic), the amino group of an amino acid accepts a proton becoming the positively charged NH3+ group while at a pH > 9.4, the carboxyl group of an amino acid loses a proton becoming the negatively charged COO- group, the overall effect being that the amino acid becomes positively and negatively charged respectively.
The million dollar question is, what happens at pH 2.2-9.4 ?. Well, the amino acid has a net zero charge. The amino acid at this state is referred to as a zwitterion. This pH at which the amino acid has no net charge is referred to as the isoelectric point of an amino acid.
Classification of Amino Acids
The different properties of the side chains of amino acids have formed the basis for its classification into different groups. It is also responsible for the distinct properties exhibited by different amino acids.
The classification of amino acids are actually very diverse and I might not necessarily have to touch every classification but I’ll be sure to do justice to this.
Amino acids can be generally classification based on the following criteria;
- Based on structure
- Based on their reaction in solution
- Based of their metabolic fate
- Based on nutritional requirement.
Classification based on structure
This class shows an unbelievable diversity. There are the simple amino acids which contain simple side chains like H and CH3. Glycine and Alanine belong to this class
There are the branched chain amino acids which contains branched side chains. Valine, leucine and isoleucine belong in this class
There are amino acids which contain sulfur in their structure and are collectively known as sulfur containing amino acids. e.g Cysteine and Methionine
There are amino acids which contain hydroxyl (~OH) groups in their side chains and collectively known as hydroxy amino acids e.g Serine and threonine.
There are amino acids which possess amide (CO-NH2) groups as their side chain. e.g. asparagine and glutamine
There are amino acids which contain two carboxyl groups in their structure e.g glutamate and aspartate
There are amino acids which contain two or more amino groups in their structure e.g lysine and arginine
There are amino acids which contain a benzene ring in their structure and are collectively known as aromatic amino acids e.g. phenylalanine and tryptophan
There are amino acids which contain cyclic side chains that are not benzene e.g. tryptophan and histidine
There is an amino acid that contains an imino acid. I guess you know this amino acid if you’ve been reading (Hint: It may or may not be proline).
Classification based on reaction in solution
Somewhere along offering chemistry in secondary school, you would have come across two special kinds of paper which turn blue or remain red and turn red or remain blue in basic or acidic solutions. Ah yes, the litmus paper. That was basically a colorimetric test for determining basicity (alkalinity) and acidity.
Some tests can however show if an amino acid is basic or acidic or even neutral. But I’m not here to talk about those tests or even highlight them at any point. I’m here to group the amino acids into these three categories.
The neutral amino acids comprise glycine, alanine, valine, leucine, isoleucine, serine, threonine, phenylalanine, tyrosine, tryptophan, histidine, proline, cysteine and methionine. Wheeeeww, you can now breathe ;).
The acidic amino acids include aspartic acid (lol), glutamic acid (lmao), asparagine and glutamine.
The basic amino acids include arginine, lysine, hydroxylysine and histidine. I’ll leave you to make the obvious observation (and no it’s not the introduction of hydroxylysine).
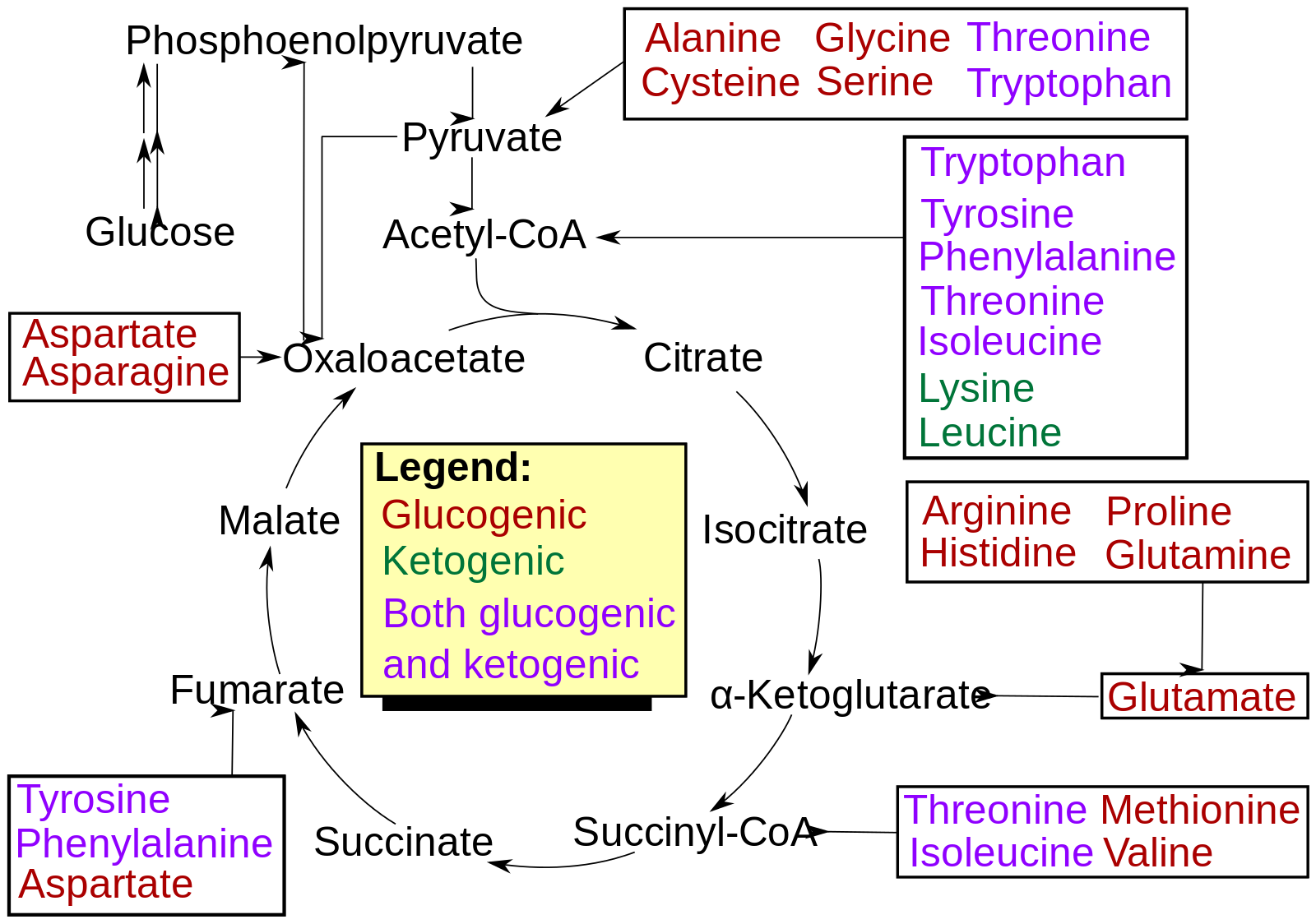
Classification based on metabolic fate
The TCA cycle (Tricarboxylic acid cycle or Krebs’ Cycle) is the hub of intermediary metabolism. Molecules from various pathways go into it and are siphoned to other pathways. It’s like the main distribution center. Amino acids go into this pathway too and this has formed a basis for the classification of amino acids.
Some amino acids are converted into TCA cycle substrates that can be used in the production of glucose and are therefore known as glucogenic amino acids. Valine, glutamate, glutamine, asparagine, aspartate, glycine, alanine, methionine, cysteine, threonine, serine, proline, arginine and histidine are fully glucogenic amino acids.
Some amino acids are converted to TCA cycle intermediates that can be used in the production of ketone bodies and are therefore known as ketogenic amino acids. Actually only one amino acid is fully ketogenic and that amino acid is Leucine
Some amino acids however are partly ketogenic and partly glucogenic. These amino acids include; phenylalanine, tryptophan, tyrosine, lysine and isoleucine.
Classification based on nutritional requirement
Some amino acids can be synthesized by the body and thus do not need to be supplied in diet. They are generally known as non-essential amino acids. However, some amino acids cannot be synthesized by the body and thus need to be supplied in diet and they are therefore considered essential
Examples of essential amino acids are; Threonine, phenylalanine, tryptophan, valine, methionine, leucine, lysine and isoleucine.
Some amino acids are only needed by children and not a requirement in adults. They are generally considered semi-essential amino acids. E.g. Histidine and Arginine
A few examples of the non-essential amino acids include; glycine, alanine, serine, threonine, aspartate, glutamate and glutamine.
Summary
The amino acids are the building blocks of proteins. They show a wide variety both in structure and function and are very essential in the processes of life. They can go ahead to form proteins through polymerization. The chemistry of proteins comes up in the next installment of this series. I hope you left here knowing a little more than you did before reading this. Till next time then.
References
Amino Acids. Wikipedia articles (accessed on April 27th, 2018)
Amphoterism. Wikipedia articles (accessed on April 27th, 2018)
Proteinogenic amino acid. Wikipedia articles (accessed on April 27th, 2018)
Chatterjea, M. N. (2012). Textbook of Medical Biochemistry. (8th Edition). Jaypee Brothers Medical Publishers. New Delhi. India. pp. 76 - 83.
Vasudevan et al. (2011). Textbook of Biochemistry. (6th Edition). Jaypee Brothers Medical Publishers. New Delhi. India. pp. 19 - 26.
Image Sources
All images are from pixabay and wikicommons licensed under creative commons and eligible for commercial use.
I'm a proud member of the steemstem community which promotes quality posts in the science, technology, engineering and mathematics fields on the steem blockchain mainly through interaction and engagement. Feel free to join us on discord here
Once again great post with full of knowledge. You are producing real quality content.
Thanks man :)
Very interesting. It seems readying about Proteins is quite more than fun than reading about Carbohydrates. LOL Nice post @kingabesh. Always looking forward to reading your posts
Thanks man. Glad you enjoyed it
Quality post, i stand for you
I wish you actually read it but thanks
Nice article!
Thanks, David
Before I studied engineering, I always thought I'd study chemistry because it was so effortless for me to understand. I was doing SS 3 chemistry in SS 1. But as I was doing my A'level we started taking biochemistry topics, I can remember vividly that amino acids was the first topic, after the second class of biochemistry. I made up my mind to study engineering. Biochemistry wasn't just meant for me. But maybe if I had met someone like you back then, I probably wouldn't have changed.
From your post it shows that you've great understanding of amino acids and biochemistry in general.
Keep up the good work brother.
Thanks man. Such an amazing comment
@addempsea. Your tales were quite similar to mine. I have always thought of being an industrial chemist before switching my way to engineering If there is one subject i love then in secondary school it was Chemistry. I so much toy with it that i was 45minutes late to an external exam for chemistry and i still passed in colors. @kingabesh Your post got my nerves on when i noticed it was amino acid.
If i can recall, if the 20 amino acids are arranged just like the 20elements. It will be very easy to distinguish between the side chain polarity( the basic polar, non polar and the acidic polar)
You have done a very good job sir. I learnt more on amino acid again today. What a post!!