Everything is A Stardust - Origin of The Elements
The periodical table of the chemical elements you see in the image below is not a regular periodic table with numbers that represent elements and a number of electrochemical properties. This time, the symbols of the elements in the squares and some letters are shown in the table. These are the letters that indicate the origins of the elements.
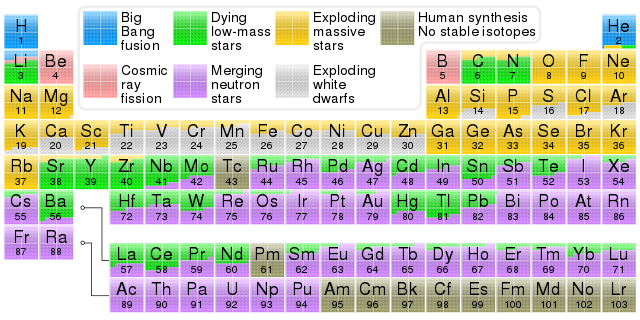
There is a reason why these tables are called periodic tables. Certain features of chemical elements follow a certain trend with a similar or more accurate expression. For example, one of the best known examples is atomic mass increase from left to right and from top to bottom of the periodic table. When we compare a weight between any two elements by looking at the periodic table, we can say that the atom to the right and/or below the other is heavier. If we are already more concerned with chemistry, we may have consistent estimates about the element by looking at the element only in its
Although the origins of the chemical elements are not dependent in a period, they are shown in the periodic table in the image above. This is a very common way of representation. For example, the physical states of the elements at room temperature in various places are also shown on periodic tables. Although the physical states of the elements at room temperature follow a certain trend, there are a few exceptions. Likewise, there is also a trend in this table where the originals are shown roughly. As you can see, the elements towards the upper left corner are colored green. And also, the elements towards the bottom right corner are seem purple. While the green-colored elements were formed in the nuclei of gigantic stars, the purple-colored elements appeared in the supernova bursts. Just by looking at the periodic table, it is not wrong that we assume that the heavy metal elements are occurred in supernovas.
There are also other colors on the periodic table. Now let’s talk briefly about these colors, or rather the corresponding origin stories.
One of the most ancient questions of mankind is the question, "Where did we come from and where do we go?" Thanks to science, which is the only tool that can answer human questions or have the greatest percentage of correct answers, answering the first question is no longer as vague as the second one.
Before beginning the subject, it is necessary to briefly explain the basic concepts such as element, atom, nucleus and isotope. Elements are pure substances which are composed of atoms of the same kind and which cannot be separated chemically more easily. There are 94 "natural" elements in the universe. The first 94 elements are produced in artificial ways, not in natural ways, but in laboratories.
Atoms are the basic units of matter. They are made up of three particles; protons, neutrons and electrons. While the weights of protons and neutrons are close to each other, the weight of electrons was considered negligible and did not contribute enough to the total atomic weight to count. Thus, the atomic weight is regarded as the sum of the weights of protons and neutrons. The numbers of protons and neutrons in the atomic nuclei are equal to each other. However, the nuclei of some atoms may contain more neutrons than the number of protons. The atoms with such nuclei are called isotopes. The increase in the number of protons in the nucleus makes it a new element. But the increase in the number of neutrons does not make it a new element because neutrons are uncharged. For this reason, isotopes are atoms with heavier nuclei of the same element. Then, the number of neutrons has increased. Neutrons are the main subatomic particles that determine atomic weight. The other is proton what I said before.
Nucleaosynthesis means forming up the new nucleus from the proton and neutrons in extraordinary places and conditions, such as the big explosion environment and star nuclei. Our topic is more related to nucleosynthesis. Nuclear synthesis should not be confused with the core fusion currently being carried out in nuclear reactors. The core fusion has become an almost routine process, as two or more atomic nuclei now form a new atomic nucleus. Today, the nucleus fusion has become almost routine as two or more atomic nuclei form a new atomic nucleus. However, nuclear synthesis is called the formation of a new nucleus by individual proton and neutrons. Nucleus synthesis is directly related to the origin of the elements. Elements continue to form in enormous quantities in various parts of the universe.
Big Bang
The single nucleosynthesis, which still does not continue, occurred in a big bang environment. Other nucleosynthesis' events that I write about below are still in progress.
In this "nucleuosynthesis" that took place between the third and twentieth minutes after the big bang, only the first two elements, hydrogen and helium elements (and isotopes), were formed. These are the two lightest elements at the same time. This explosion, which is indirectly the "big mother" of all other elements, is regarded as the beginning of the universe we know today. There are various scientific theories about pre-explosion, as well as other philosophical and religious explanations. However, the main subject we talked about is not the big bang. Our main subject is starting from the third minute of the big bang.

The first 20 minutes of the big bang formed a small amount of the Lithium-7 isotope, a lithium-stable isotope. Also, a small amount of Beryllium-7 isotope was formed. However, since this isotope is an unstable isotope, it is degraded to Lithium-7 and hydrogen-3 isotopes. Lithium and beryllium are also the two lightest elements after hydrogen and helium.
The exception of these small lithium and beryllium isotopes is not shown in the table. However, if you would like to touch something directly from the big bang, and helium or hydrogen because of they are gases, if they do not give you the feeling you want, you can taste the feeling of touching something that is left over from the big bang by touching the lithium element. Some of the lithium in the nature left over from the big bang. The other part, you will read soon, was formed up as a result of cosmic ray fragmentation.
Fragmentation of Cosmic Rays
Lithium, beryllium, and boron elements are formed by the formation of a new nucleus of protons and neutrons, which are caused by high-energy cosmic rays striking atoms in the environment and causing them to fly through the nucleus. Nearly all of the helium-3 isotopes in the world are formed in this way. However, the helium-3 isotope forms very little helium in the universe. Due to the cosmic rays, some small amounts of aluminum, carbon (especially the famous carbon-14), chlorine, iodine and neon isotopes are also formed. The nuclei of these rare isotopes are called cosmogenic nuclides. Scientists can make geological determinations on the related world rocks or meteorite specimens from the path of cosmic nuclides and other isotopes of the element formed by cosmic rays.
These rare and exceptional isotopes are not shown in the first image and only the lithium, beryllium and boron elements, which are caused by very large quantities, are labeled in blue. As we noticed, cosmic rays in the periodic table it is shown as the reason of the occurrence of the three elements and the atomic numbers of these three elements are 3,4 and 5. If we want to add some of the rare isotopes we mention, we can reach 6, 7 and 8. However, we can not go further. This also means, we need more energy than high-energy cosmic rays to form heavier atoms, ie the desired temperature for more protons brought together in the same core. It seems logical that there are incredible temperatures and pressures to combine more protons and neutrons for this job.
Which furnace can do this job? Of course, the big nuclear furnaces in the sky. Maybe it's not so romantic like that, but in fact the stars deserve more romanticism as nuclear furnaces that increase elemental diversity rather than being a witness of a romantic night.
Star and Supernova Nucleosyntesis
If we look at the periodic table, we may notice that the vast majority of the elements is formed up in star nuclei and supernova explosions. Almost all of the bottom right corner of the periodic table is pink in color. This shows us that heavy atoms only form in the supernova explosions. This shows us that heavy atoms only formed in the supernova explosions. As the atomic weights decrease, the green and yellow colors take place on the table. These atoms are formed in the stars.
Star and supernova nucleosyntheses are astrophysical topics that include a set of calculations that are challenged even by specialists. Let's continue with the information that includes only the principles of formation, without going too far away from the chemistry of the subject.

Stars are huge plasma pools that stand together by their mass-gravity. Just like the evolution of living things, the evolution of stars has been a matter. The first stars were formed when the first two elements came together after the big explosion. The explosion of these stars gave birth to star systems with second-generation stars and planets with heavier atoms flying around them, their satellites and other small celestial bodies. Our solar system is one of them. Although the stars seemed too close to the naked eye to be indistinguishable, they were separated into various species and classes by astrophysicists. A star's diameter, color, and brightness give an idea of the age of the star at first glance, but you can have more information about it from analyzing the light from the star world to the spectrum.
The gravitational attraction of the stars to themselves is the chief actor of the evolution of the stars and the formation of the element we will soon talk about soon. We can compare stars to balloons. As the atmospheric pressures down, the air inside the balloon will expand itself to equalize with the atmospheric pressure. However, when we increase the external pressure, the external pressure will be followed by the pressure and the balloon will shrink until the internal pressure is equal to the external pressure. We're talking about a pressure balance here. The balloon will inflate spontaneously and its elastic structure will not withstand that tension and will explode at low altitudes of atmospheric pressure. In the stars, this equilibrium is not pressure balance; the nuclear reactions are the outgrowth from the final center and the collapse to the center due to mass. While the core reactions want to expand the star, its mass wants to shrink the star towards its center.
The pressure and temperature in the star nuclei are so high that the helium and hydrogen nuclei are close to each other, almost intertwined. This high pressure and temperature is too high for us to imagine with our everyday experiences. However, this strange balance within the star prevents it from producing all the elements in its core. This temperature and pressure can only produce up to the iron element. Because the force of the star's collapse of its center is no longer strong enough to bring the heavier atomic nuclei to the square. It has gained core pressure for expansion.
Other extraordinary requirements are required for atoms heavier than iron. This is supernova explosions. We can continue to simulate supernovas like balloons, exploding due to internal and external pressure balance. Especially the mass stars, in their evolution, as they age, as the elements in the kernel form, the balance is completely distorted, exploding out of the nuclei to the surface and distributing the heavy elements of its content of the space. The supernova explosions have been divided into various groups by astrophysicists, taking into account their various characteristics.
The supernova explosions are now preparing an environment that will allow the formation of heavier nuclei from the iron. With the explosion of a star as a supernova, now 94 natural elements are formed. This environment is the center of new stars to be born with a sense. From here stars and celestial bodies will spin around them.

The abundance of the elements in a solar system and their ratio to each other depends on the content of the cloud in which it is born and the content of the star forming that cloud. Looking at the abundance of the elements in our world and our solar system, we can learn about the content of the first star that gave birth to us, and even our sister solar systems.
The fact that life in the world is carbon origin is due to the fact that carbon is the lightest element produced in the stars, and as a result, it is found in abundance and scattered around. Or is carbon the only logical building block for a living, as it can create millions of different compounds? Again, oxygen and nitrogen, which are essential for life, are scattered to the nose of the solar system because they are light elements, so important for the emergence of vitality? Or could there be other elements instead of oxygen and nitrogen? Only the sum of oxygen, carbon, nitrogen, and hydrogen accounts for 91 percent of our body weight. Is there anything of interest in this ratio, as the elements of the bet are light and are abundantly present?
Of course the answer to these questions is not easy. There are many things that we do not know, can not calculate, we will learn when calculating. However, the periodic table can help us give a general direction to our way of going. Life is chemistry. Although the elements in the same group do not have to resemble color, smell and other physical properties, their chemical behavior is similar in the periodic table. For example, we can remove oxygen from the water and replace it with sulfur. Because in the periodic table these two elements are bottom the each other. Or, using silicon instead of carbon, we can produce silicon polymers similar to carbon polymers. Are there any silicon-based creatures developed in hydrogen sulphide elsewhere in the universe? Trying to respond to these questions can not go beyond the brain gymnastics. We now know exactly the origins of the elements. However, the "answer misses" that science has responded to today are a good reference for science, and perhaps one day it will answer these questions.
I want to end my article with the famous sentences of Astronomer Dr. Carl Sagan.
“The cosmos is within us. We are made of star-stuff. We are a way for the universe to know itself.”

References
- https://cosmicopia.gsfc.nasa.gov/
- http://www2.lbl.gov/abc/wallchart/chapters/10/0.html
- https://en.wikipedia.org/wiki/Nucleosynthesis
- https://www.bibliotecapleyades.net/ciencia/ciencia_originelements.htm
- https://en.wikipedia.org/wiki/Big_Bang_nucleosynthesis
- https://en.wikipedia.org/wiki/Stellar_nucleosynthesis
upvote for me please? https://steemit.com/news/@bible.com/6h36cq
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Yaser from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 11 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 20 SBD worth and should receive 160 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePigThis post received upvote from @tipU :) | Voting service | For investors.