(The History of Chemistry) The Haber Process: A Reaction Few Know About But All Are Dependent On
Today I will take a break from my usual posting style of summarizing new research publications (besides who could compete with the post that user @irime put out this week! If you haven't checked that post out, I recommend it!). Instead, let us take a step back in time to the late 1890's to early 1900's and discuss the development of an important chemical reaction known as the Haber process (or Haber-Bosch process if you want to credit its further scale up).

The Haber Process
Quite simply the Haber process is a chemical reaction which allows for the production of Ammonia (NH3) from Hydrogen and Nitrogen from our atmosphere:
From looking at it you might be thinking, okay.. so? These elements are in the air so the ammonia must be able to form easily (after all you might note that the Gibbs free energy is negative! [4] That means the reaction is spontaneous!). Unfortunately this was not the case and the discovery of how to appropriately perform this reaction revolutionized the world as we know it.
Wait... why doesn't the reaction proceed? First principles says it should damn it!
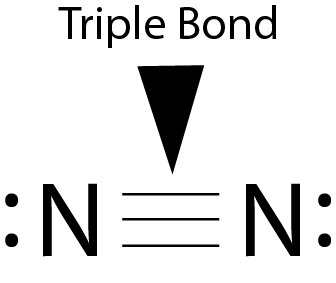
Nitrogen is not a particularly reactive element, as the elemental form (N2 is held together very very tightly by a triple bond, see image to the right). In order for this reaction to proceed we have to break a part that triple bond of nitrogen. To do this, one can heat the nitrogen up to about 3000 °C!
Unfortunately for us, the nitrogen fixation reaction above is exothermic meaning that heat is one of the products. Doubly unfortunate for us the reaction is an equilibrium reaction, this means that if we add more heat, it will reduce the yield (because we are shifting the equilibrium back toward the reactants side as we are adding in more of a product).
This leaves us with a problem, if we want the reaction to work we must raise the temperature up to break that nitrogen bond. But if we raise the temperature up and break the nitrogen bond then the reaction wants to go backwards and we don't get any product! This was where a man named Fritz Haber came in!
Fritz Haber

Fritz Haber was a German scientist who was working at the University of Karlsruhe in Karlsruhe, Germany (I am sure you figured the location out, but sometimes its fun to state the obvious). He was a great physical chemist and realized the only way to circumvent the issues with the equilibrium of this reaction would be to find a catalyst that would lower the energy required to split the nitrogen triple bond.
And identify a catalyst he did! Haber identified that the metal osmium could serve as a sort of scaffold to allow the underlying steps of the ammonia production reaction to proceed. [6]
This scaffold reduced the activation energy necessary to break apart the Nitrogen drastically reducing the required temperature and allowed for the reaction to proceed through several steps:
N2H2 + H2 --> N2H4
N2H4 + H2 --> 2NH3
The actual detailed inorganic mechanism by which the Osmium works, is a bit complicated but if you are interested you can see a bit more here.
This catalyst was later optimized from Osmium to Iron and Potassium Hydroxide (as an activator of the iron surface). [8]
Now thanks to the identification of a chemical catalyst, Haber was able to perform the reaction under high pressure and only mildly high temperatures (~500 - 600 K or 227 - 327 °C).
But How To Separate The Gasses?
Okay so we have performed this reaction and made Ammonia gas (hooray?)... but there is still some left over hydrogen and of course atmospheric nitrogen to contend with, how can we separate the ammonia away? The answer to this is simple. Cool the gasses down, the ammonia will precipitate out into a liquid form at a much much higher temperature (-33 °C) then either Nitrogen (-195 °C) or Hydrogen (-252.9 °C).So cool the gas and the ammonia will turn into a liquid first!
Why Was A Reaction To Produce Ammonia Such A Game Changer?
Fertilizer! The worlds population was growing in the late 1800's and it was becoming increasingly difficult to produce enough food to feed everyone. To really ramp up food production farmers were turning to the use of a lot of fertilizer! This fertilizer required a nitrogen source like say... ammonium nitrate.
So the discovery that Haber made in being able to chemically produce ammonia under relatively gentle conditions using a catalyst was a HUGE HUGE deal for the world.
His discovery here was one of the major contributors to the great success that we humans have had on earth! It allowed our populations to grow and people to remain fed! Granted as humanity has continued to expand we have found new challenges in feeding people, but we would not have gotten to where we are with out the discovery that Haber made.
Coincidentally, Haber won a Nobel Prize for this work (and he should have!).
You may not spend much time thinking about chemical reactions like this. However I hope that this short post has illustrated to you how complicated something as simple as producing ammonia can be, and also how much of our quality of life is reliant on simple chemistry such as this!
Sources
Text Sources
- https://en.wikipedia.org/wiki/Haber_process
- http://www.ias.ac.in/article/fulltext/reso/007/09/0069-0077
- https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Equilibria/Case_Studies/Haber_Process
- http://surfguppy.com/thermodynamics/caluclate-gibbs-free-energy-nitrogen-and-hydrogen-to-create-ammonia/
- https://en.wikipedia.org/wiki/Fritz_Haber
- http://www.spaceflight.esa.int/impress/text/education/Catalysis/Commercial.html
- Nitrogen Fixation
- https://www.researchgate.net/publication/256293116_AN_IRON_CATALYST_FOR_THE_SYNTHESIS_OF_AMMONIA_IMPREGNATED_WITH_POTASSIUM_HYDROXIDE
- https://passel.unl.edu/pages/informationmodule.php?idinformationmodule=1130447045
- https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1918/
Image Sources
All Non Cited Images Are From Pixabay.com, Flickr.com, Pexels.com, or Wikipedia.com And Are Available For Reuse Under Creative Commons Licenses
Any Gifs Are From Giphy.com and Are Also Available for Use Under Creative Commons Licences
If you like this work, please consider giving me a follow: @justtryme90. I am here to help spread scientific knowledge and break down primary publications in such a way so as to cut through the jargon and provide you the main conclusions in short (well compared to the original articles at least!) and easy to read posts.
SteemSTEM
Secondly, please consider supporting the @steemstem project. SteemSTEM is a community driven project which seeks to promote well written/informative Science Technology Engineering and Mathematics postings on Steemit. The project not only curates STEM posts on the platform through both voting and resteeming, but also re-distributes curation rewards as STEEM Power, to members of Steemit's growing scientific/tech community.
To learn more about the project please join us on steemit.chat (https://steemit.chat/channel/steemSTEM), we are always looking for people who want to help in our quest to increase the quality of STEM (and health) posts on our growing platform, and would love to hear from you!

Very well merited Nobel Prize mate! I'm just thinking about all the good this discovery made to humanity! Simply amazing!
I never knew that ammonia was made like that, i'm always learning so much from your full of info post.
Thanks so much!
@progressivechef
Thank you so much for your kind words! I truly appreciate having you as a reader man. You always have something productive to say about my posts. I am going to reward you today for your going out of your way to do me this kindness.
Oh wow! This is just unbelievable man! Thank you so much. I really enjoy reading and growing my knowledge on subjects that I am not too good in.
With this upvote you contributed greatly/sponsored for a prize in my cooking contest.
Cheers!
I voted on that post for you too. How about this comment as well. If you are going to give it out to others as prizes, then I'm all for helping :)
And here you go 2 more prizes sponsored! This is just amazing...I must have really done something great this morning to be rewarded like this!
Each week I share 31SBD as prizes to the top 7 in the cooking contest man. I already have @canadian-coconut and @donkeypong helping me on my daily post, you can see their votes on all my posts. I just love the original foodies and I am supporting quite a lot of them with this contest.
Thank you thank you thank you!
I wish you success always mate!
Nah, you're just being you. I like genuinely nice people, and you clearly fit that! It's easy to support someone who is kind to others.
I love you and love your publications I want to be like you and I would be grateful that if you support me, I love you, O Great
Woah, take it easy.
Hahahah! You are getting some love too...This comment made me laugh so much mate!
Haha I didn't know that it was started with osmium !!
Yeah! The Nobel Prize for this year is approaching XDDDDD
Yep, he also did it with uranium.
Scientist is always crazy.......
It was just sounds as crazy as using thallium to catalyze Suzuki coupling: how can one come up with such a crazy idea to use thallium salt........
Beer and a lot of late nights ;)
The zombie proofreader in me cringes when I stumble over "tripple" in this wonderfully conversationally-written post about Haber's work...while it would not have been the exclusive solution to synthetic fertilizer, even more than a century ago, it's certainly the most economical one that scales in production, and the only one that would have been politically and socially practical.
Ugh, I can't spell!
Edit: I hate it when I do things like that. I think I have fixed it.
Thanks for reading the post. I think Haber's contributions to society were massive and despite that few even know who he was. I suppose its because ammonia isn't exactly the sexiest of compounds/topics.
Indeed, well said.
That particular comment was leading, intended to spur some analytical thinking in at least one other person as to some of the external ("worldly") constraints on such an important development (from the theoretical and research aspects.)
How much impact some discovery can make! I learned a little bit about chemistry in high school but never related to real life events. I mean, we were doing the practise, and learning the theories, but our teacher didn't explain the 'why' such and such was so important, or what a certain experiment we were doing, was good for. Maybe explaining that would make chemistry classes to complex, maybe it was simply a matter of time available, maybe it is our Ducth system. I for sure learned something today!
Its usually this. They have so many fundamental concepts to cover, that there just isn't enough time to put the concepts into a real life context. Would doing that make things more interesting for the students? Probably yeah. However, then we would need like 4-5 years of chemistry to cover the material :D
You have a point!
I haven't been here for quite a while but it's very good to see that you and your science articles are still going strong. You never cease to amaze. :-)
There is just one tiny, little, little typo in the article above. I'm sure you can find it yourself: "Fritz Haber was a German scientist who was working at the University of Karlsruhe in Karlshrue, Germany".
Anyway, thank you so much for holding up the banner of science on this platform and keep up the good work!
:( oh man, another incredibly stupid typo in this post. Thanks for pointing it out.
Don't be so hard on yourself. The article is awesome!
Wow this post was interesting, its amazing to see how far humanity has come forward and definitely it was a well deserved nobel prize
Indeed, thanks for taking the time to read it and for your comment! There are so many nobel prize winners over the years, and yet how many do any of us really know about? A small handful at best. Yet if you were to look into the work that these people achieved you might think... how is this person not a household name!!??? It's a question I often ask my self, I know so much about scientists of old, Newton, Salk, Copernicus, Galileo, but when I think about modern day scientists ... I only know a few! Why is that...?
Steem meets chemistry lol
Quite interesting this story, it is always good to know a few topics totally opposite to those that you have to have daily, the chemistry is very interesting and is one of the most important branches of science, at least in my area medicine la we use daily and as well as in others more like the physics, great estimated contribution @justtryme90
Thanks for reading.
you are welcome, my friend, for such excellent publications @justtryme90
Haber process was the very common question being asked during the science viva and practical during the exam.
What did they ask about it?
Just explain the Haber Process In detail and write the reaction involved in it.
They had you explain why high pressure is used and things like that? :)
Lots of basic, fundamental chemistry can be discussed with this simple equilibrium.
Yes this was well explained in the books.
This brought to reminiscence my high school memory. The first time I heard about harber process, in SS2 (Senior Secondary 2), the name sounded quite funny. We had to nickname our Chemistry Uncle "Harber Process" because that was the first topic he taught us :)
HAH! Thats pretty funny.
I just noticed this post today (week-end times, remember :p). This was totally new to me (I didn't know anything about that). What is the exact contribution of Bosch? (I could google it but I have unfortunately no time there, and perhaps you know in 5 words :p
Bosch came up with the way to scale the reaction up to actual industrial production levels. Equally important and also Nobel prize worthy.