Sucrose and Cellulose Synthesis
Going with the phloem
The cells of the mature, actively photosynthesizing plant leaf are a major "source" of sucrose. Sucrose generated by these cells is actively loaded into the phloem (a conduit of cells, inter-connected by seive plates) that surround the water-transporting xylem tissue. Phloem tissue delivers the sucrose to "sinks" -- tissues/organs of the plant that are non-photosynthetic (e.g. roots, flowers, developing fruit, and young expanding leaves that have not yet reached full photosynthetic potential) -- often over long distances. The "sinks" consume sucrose, using it to derive carbon skeletons for starch synthesis, energy (via glycolysis and the citric acid cycle), and as an immediate precursor for the synthesis of cellulose and hence cell wall biosynthesis. The sucrose concentration in the phloem at the "source" leaf is typically high when photosynthesis is in progress (i.e. the solute potential of the phloem at the source is low). The sucrose concentration in the phloem at the "sink" tissue is low (i.e. the solute potential of the phloem at the source is high) due to sucrose consumption. Thus, there is a solute potential (pressure) difference between "source" and "sink" which facilitates sucrose flow from "source" to "sink". Sucrose tends to flow to the most active "sinks" (i.e. the sinks which have the most rapid rates of utilization/consumption of sucrose). Removal of a sink (e.g. a developing fruit) automatically redirects the flow of sucrose to other sinks.
I would like to recommend this video that explains phloem translocation from "source" to "sink":
Explanation of transport of sugar via the phloem in plants
by Dan Rott
Published on Apr 9, 2015
and the following web site for a more detailed description of the process: IB STUDY BUDDY. 9.2 Transport in the Phloem of Plants.
So how is sucrose synthesized in leaves, and how is it consumed in sinks?
Sucrose synthesis
Returning to the image from Carbohydrate Metabolism in Plants - Overview, I would like to focus on the upper-right quadrant:
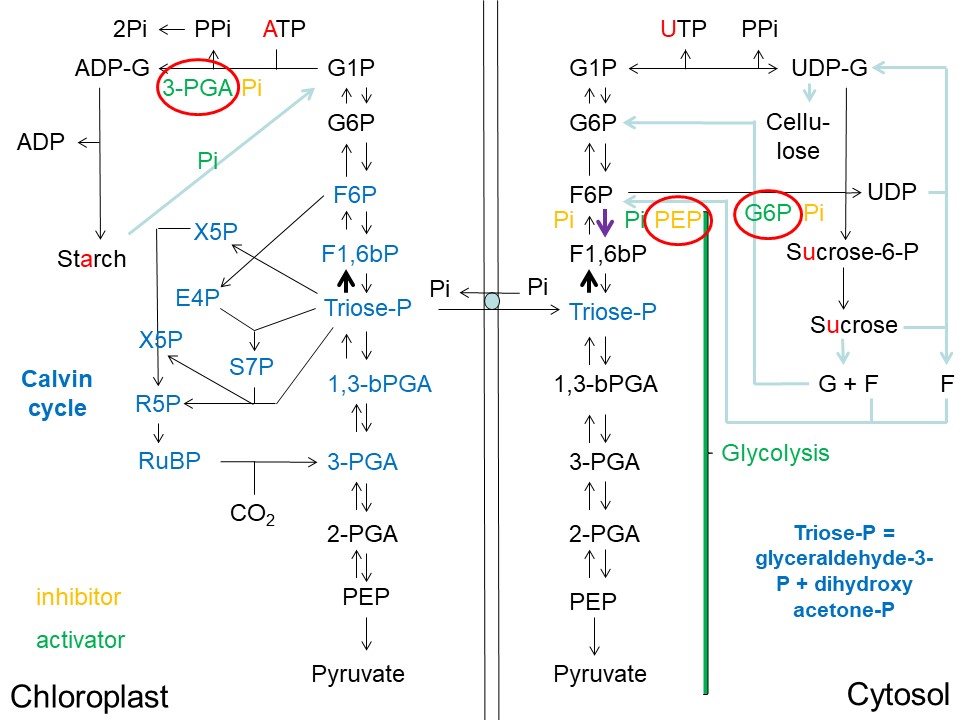
Overview of Starch, Sucrose and Cellulose Synthesis in Plants and its Regulatory Architecture (D. Rhodes)
Abbreviations used: ADP = adenosine-diphosphate; ADP-G = ADP-glucose; ATP = adenosine-triphosphate; E4P = erythrose-4-phosphate; F = fructose; F1,6bP = fructose-1,6-bisphosphate; F6P = fructose-6-phosphate; G = glucose; G6P = glucose-6-phosphate; G1P = glucose-1-phosphate; Pi = inorganic phosphate; PPi = pyrophosphate; 3-PGA = 3-phosphoglycerate; 2-PGA = 2-phosphoglycerate; PEP = phosphoenolpyruvate; 1,3-bPGA = 1,3-bisphosphoglycerate; RuBP = ribulose-1,5-bisphosphate; R5P = ribulose-5-phosphate; S7P = sedoheptulose-7-phosphate; sucrose-P = sucrose-phosphate; triose-phosphate = glyceraldehyde-3-phosphate + dihydroxyacetone phosphate; UDP = uridine-diphosphate; UDP-G = UDP-glucose; UTP = uridine-triphosphate; X5P = xylulose-5-phosphate.
Triose-phosphate (triose-P) synthesized via the Calvin cycle in the chloroplasts of the "source" leaf, exits the chloroplast (into the cytoplasm) via the triose-phosphate/phosphate translocator in exchange for phosphate (Pi). As in the chloroplast, the conversion of 2 x triose-P --> F1,6bP in the cytosol is dependent upon the square of the triose-P concentration (see bold black arrow in the upper-right quadrant of the image above) (Jones et al. (2002)). If the cytoplasmic Pi concentration is high, the conversion of triose-P to F1,6bP is inhibited, and the reverse reaction F6P --> F1,6bP (catalyzed by phosphofructokinase) is activated (see bold purple arrow in the upper-right quadrant of the image above) (Jones et al. (2002)). The latter enzyme is the principal regulatory enzyme of glycolysis in plants and directs flow to glycolysis in the cytoplasm when cytoplasmic Pi concentration rises. However, a rise in phosphoenolpyruvate (PEP) concentration in the cytoplasm will slow down this glycolytic flux by inhibiting phosphofructokinase (Jones et al. (2002)).
If PEP levels rise and Pi levels fall in the cytoplasm, the conversion of 2 x triose-P --> F1,6bP --> F6P --> G6P is favored over glycolysis. Rising G6P levels and falling Pi levels then favor the conversion of F6P to sucrose-6-phosphate (sucrose-P). G6P is the precursor of G1P, which in turn is converted to UDP-glucose (UDP-G). UDP-G in turn combines with F6P to form sucrose-P and UDP in the reaction catalyzed by sucrose-phosphate synthase (discussed in more detail below).
UDP-G is formed from G1P and UTP in the reaction catalyzed by UDP-G pyrophosphorylase:
This reaction is similar to that catalyzed by ADP-G pyrophosphorylase in the chloroplast/plastid and discussed in my previous post on Starch Synthesis and Catabolism:
While the pyrophosphate (PPi) produced in the chloroplast/plastid is rapidly converted to 2 x Pi by a pyrophosphatase (rendering the ADP-G pyrophosphorylase reaction essentially irreversible) this pyrophosphorylase activity is not present in the cytoplasm. While ADP-G pyrophosphorylase is the key point of regulation of starch biosynthesis in the chloroplast/plastid, the major control of sucrose biosynthesis in the cytoplasm does not occur at the UDP-G pyrophosphorylase step. Rather, control of sucrose biosynthesis resides mostly at the step catalyzed by sucrose-phosphate synthase (SPS)(see Sucrose-phosphate synthase - Wikipedia):
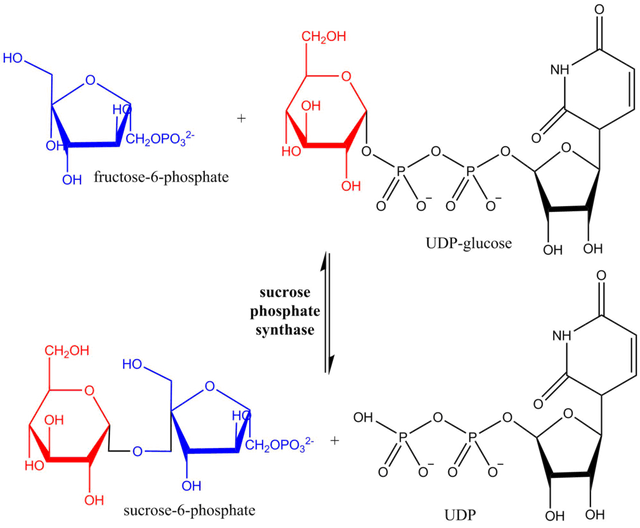
Reaction scheme showing hexosyl group transfer from UDP-glucose to fructose-6-phosphate catalyzed by SPS. Image Source:
Sucrose-phosphate synthase (SPS) is the key regulator of sucrose synthesis in plants, and it is controlled in a highly sophisticated manner (Huber and Huber (1996)). First, this enzyme is activated by G6P and inhibited by free Pi. Thus, its activity is critically dependent upon the ratio of G6P:Pi. Second, the enzyme can be interconverted between active and inactive forms via dephosphorylation and phosphorylation of specific serine residues of the enzyme. Phosphorylation of the enzyme is catalyzed by a specific protein kinase, and dephosphorylation of the enzyme by a specific protein phosphatase. The protein kinase and phosphatase that phosphorylate and dephosphorylate sucrose-phosphate synthase are in turn regulated by the concentrations of G6P and Pi. Pi inhibits the protein phosphatase (SPS-PP) that converts sucrose-phosphate synthase into its active form. G6P inhibits the kinase that converts sucrose-phosphate synthase into its inactive form (Huber and Huber (1996), Sugden et al. (1999)). Moreover, the expression of the protein phosphatase (SPS-PP) that converts sucrose-phosphate synthase into its active form is diurnally regulated, with most active expression in the light (Huber and Huber (1996), Jones and Ort (1997)). This ensures that when G6P levels are high in the cytoplasm, and Pi levels are low, flux is diverted towards sucrose biosynthesis in the phosynthetically active leaf cell. Regulatory proteins (14-3-3) proteins appear to be involved in this regulation of SPS (Moorehead et al. (1999)). Chilling stress (Jones et al. (1998)) and osmotic stress (Toroser and Huber (1997)) can also significantly influence this intricate control mechanism.
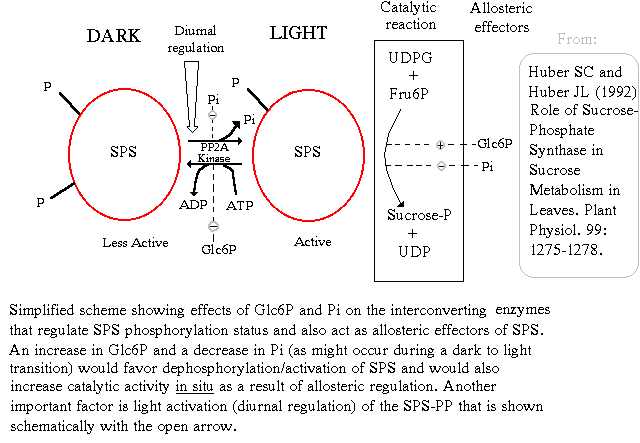
Sucrose-phosphate synthase regulation by phosphorylation/dephosphorylation, G6P and Pi (D. Rhodes). Image Source:
Sucrose-P (the product of the catalytic action of sucrose-phosphate synthase) is simply dephosphorylated by sucrose-phosphate phosphatase to form sucrose, rendering the sucrose-phosphate synthase reaction essentially irreversible (Echeverria et al. (1997)).
If we look back at my previous post on Starch Synthesis and Catabolism, we can now begin to see why the starch-deficient shrunken-2 (sh2) mutant of maize (supersweet sweet corn) accumulates sucrose. The failure to utilize G6P in starch biosynthesis in the amyloplast of the endosperm cells leads to accumulation of G6P which then activates sucrose-phosphate synthase in the cytoplasm.
Cellulose synthesis
Sucrose is the immediate precursor for the glucose moieties of cellulose. The enzyme sucrose synthase catalyzes the reversible conversion of sucrose + UDP <--> fructose + UDP-G. The name "sucrose synthase" refers to its activity in the reverse direction. In vivo it probably mostly functions in formation of UDP-G from sucrose + UDP to provide the UDP-G that serves as the substrate of Cellulose synthase (UDP-forming) - Wikipedia:
The catalytic activity of cellulose synthase produces UDP which can then be reused by sucrose synthase to regenerate UDP-G. Cellulose synthase is located in rosette structures embedded in the plasma membrane surrounding the cytoplasm. As the glucose moieties are added to the growing [(1->4)-ß-D-glucosyl]n chains, these chains are extruded to the outer surface of the plasma membrane.
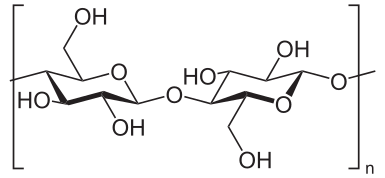
Cellulose, a linear polymer of D-glucose units (two are shown) linked by ß(1->4)-glycosidic bonds. Image Source:
These glucose chains are then wrapped into microfibrils that ultimately form the cellulose fibres of the cell wall (see Cellulose - Wikipedia).
Sucrose synthase is likely closely associated with cellulose synthase on the inner/cytoplasmic surface of the plasma membrane.
The fructose generated by the concerted action of sucrose synthase/cellulose synthase:
UDP-G + [(1->4)-ß-D-glucosyl]n ---> UDP + [(1->4)-ß-D-glucosyl]n+1
can be re-phosphorylated to F6P to re-enter the hexose-phosphate pool.
Sucrose has other metabolic fates
Sucrose can also be metabolized to fructose (F) + glucose (G) by the enzyme invertase. The resulting F + G can in turn be phosphorylated to re-enter the hexose-phosphate pool and be either metabolized towards triose-P in glycolysis, or back to sucrose and cellulose biosynthesis (Jones et al. (2002)), depending upon the levels of free Pi, G6P, PEP and triose-P in the cytoplasm. Alternatively, G6P may be imported into the amyloplast of the sink tissue cells and converted to starch (Starch Synthesis and Catabolism).
In my next post I plan to address glycolysis.
References
Additional Reading
Click on the following links for additional references on sucrose-phosphate synthase, sucrose-phosphate phosphatase, sucrose synthase, UDP-glucose pyrophosphorylaseand cellulose synthase.
As before, please feel free to ask questions or add comments below.
Hi there, I am not sure if some images in your post are free from copyright, can you please have a look? Thanks
Image of cellulose is from Wikipedia. Image of SPS phosphorylation/dephosphorylation was created by myself, redrawn from Huber and Huber (1992). Is it this image that you are concerned about? Image of sucrose-phosphate synthase substrates and products was from Wikipedia. Image of overview of carbohydrate metabolism was by myself. The first image of phloem transport appears in many places on the internet when you search for "phloem transport of sucrose" ... is it this image that you believe is copyrighted?
Especially the first image, it doesn't matter how many times it appears on internet, what matters is that the original author of the image gives it the right attribution for its reuse. Some journals have a permission page, often it's automatic and it takes literally 30 seconds to ask for permission from the journal, I had a similar issue myself and I posted a screenshot of the permission at the end of my post
Thank you for this information. I shall replace the first image if I cannot find its original source and obtain permission. Much appreciated!
I know it's a picky thing but it's for your own good ;)
I hope that the modifications just made will be satisfactory. Thanks again.
Brillant. I like how you cogent explained the sucrose metabolism and its reversible reaction in the cytoplasm as a result of the accumulation of G6P which may function as a catalyst. Nice writeup @davidrhodes124, as usual, more.
Thank you!