Starch Synthesis and Catabolism
In my last post, I gave an overview of plant carbohydrate metabolism highlighting the general architecture of the metabolic pathways in the chloroplast and cytoplasm of plant cells and pointing out that the concentration of phosphate (Pi) plays a critical regulatory role in directing the flow of carbon through the different branches (Carbohydrate Metabolism in Plants - Overview). For simplicity, I did this without citing any references, but probably this was a violation of #steemstem rules, and for this I apologize.
Here I would like to focus specifically on the upper-left quadrant of the "Overview" image dealing with starch synthesis and catabolism in the chloroplast:
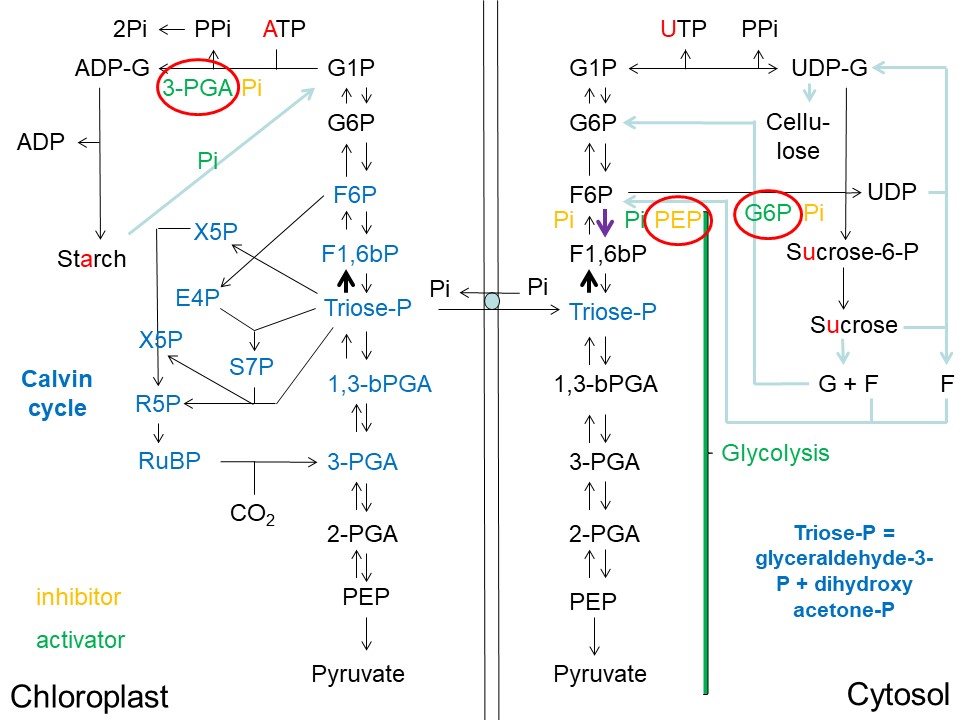
Overview of Starch, Sucrose and Cellulose Synthesis in Plants and its Regulatory Architecture (D. Rhodes)
Abbreviations used: ADP = adenosine-diphosphate; ADP-G = ADP-glucose; ATP = adenosine-triphosphate; E4P = erythrose-4-phosphate; F = fructose; F1,6bP = fructose-1,6-bisphosphate; F6P = fructose-6-phosphate; G = glucose; G6P = glucose-6-phosphate; G1P = glucose-1-phosphate; Pi = inorganic phosphate; PPi = pyrophosphate; 3-PGA = 3-phosphoglycerate; 2-PGA = 2-phosphoglycerate; PEP = phosphoenolpyruvate; 1,3-bPGA = 1,3-bisphosphoglycerate; RuBP = ribulose-1,5-bisphosphate; R5P = ribulose-5-phosphate; S7P = sedoheptulose-7-phosphate; sucrose-P = sucrose-phosphate; triose-phosphate = glyceraldehyde-3-phosphate + dihydroxyacetone phosphate; UDP = uridine-diphosphate; UDP-G = UDP-glucose; UTP = uridine-triphosphate; X5P = xylulose-5-phosphate.
Starch Synthesis and Degradation in Chloroplasts of Plant Leaves
The key enzyme regulating starch biosynthesis in plants is the enzyme catalyzing the conversion of ATP and G1P into PPi and ADP-G. This enzyme is called ADP-glucose pyrophosphorylase (also known as glucose-1-phosphate adenylyltransferase). In principle this enzyme is freely reversible:
However, chloroplasts/plastids of plant cells express an enzyme called pyrophosphatase that rapidly converts PPi to 2 x Pi, rendering the above reaction essentially irreversible (Jones et al. (2002)).
ADP-glucose pyrophosphorylase is activated by 3PGA, the first product of CO2 fixation by RuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase), and inhibited by inorganic phosphate (Pi). The ratio of 3PGA:Pi is a crucial regulatory parameter for this enzyme and is directly related to its activation state (Kleczkowski, 1999).
Thus, in an illuminated chloroplast when the light reactions of photosynthesis occur, ATP levels rise, Pi levels fall, 3PGA levels rise due to activation of RuBisCO (Michelet et al., (2013)) and conditions are set for starch accumulation, particularly if Pi levels are low in the cytoplasm. Low Pi levels in the cytoplasm would permit retention of triose-phosphates (triose-P) in the chloroplast rather than export to the cytoplasm in exchange for Pi via the triose-phosphate/phosphate translocator.
The enzyme catalyzing the reaction triose-P --> F1,6bP (FBA, fructose-1,6-bisphosphate aldolase) uses 2 x triose-P as substrates. Other enzymes in the Calvin cycle use only a single triose-P as substrate. Thus, the reaction 2 x triose-P --> F1,6bP depends upon the square of the triose-P concentration, and is more sensitive to changes in triose-P levels than other enzymes consuming triose-P in the chloroplast. Build-up of triose-P in the chloroplast therefore favors increased flux to F1,6bP (see bold black arrow in the upper-left quadrant of the image above) (Jones et al. (2002)).
Once ADP-glucose (ADP-G) has been formed, the enzyme starch synthase then adds the ADP-G to a growing chain of glucose residues, releasing ADP and forming amylose. The glucose molecules are linked via 1,4-alpha glycosidic bonds in the unbranched starch, amylose. Starch branching enzyme may then introduce 1,6-alpha glycosidic bonds between these chains, creating the branched starch, amylopectin.
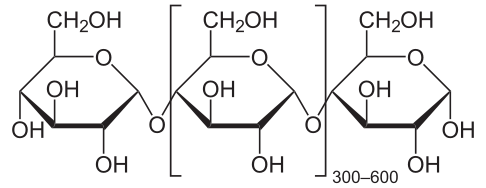
Structure of the amylose molecule. Image Source:
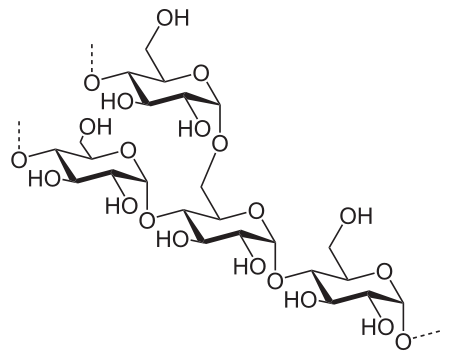
Structure of the amylopectin molecule. Image Source:
Starch is typically synthesized during the day by leaves and stored as granules in the chloroplast stroma. At night, starch is then catabolized and used as an energy source (Starch- Wikipedia). Notably, starch catabolism is dependent upon free Pi which typically increases in chloroplasts in darkness when the light reactions of photosynthesis cease. "Starch phosphorylase catalyzes the reversible transfer of glucosyl units from glucose-1-phosphate to the nonreducing end of alpha-1,4-D-glucan chains with the release of phosphate" (Rathor et al. (2009)). At night this enzyme essentially catalyzes the reaction:
A number of glucan, water dikinases (GWDs) may also be involved in starch phosphorylation, facilitating its degradation (Hejazi et al. (2012)).
Starch Synthesis and Degradation in Plastids (Amyloplasts)
In roots and tubers, and in non-photosynthetic plant organs such as seeds, the plastids do not develop into chloroplasts capable of photosynthesis. They lack expression of enzymes required for chlorophyll biosynthesis, and do not express a full complement of Calvin cycle enzymes. Yet they are capable of starch synthesis, relying upon sucrose as a carbon source, imported via the phloem from photosynthetic leaves (I will talk more about sucrose biosynthesis in leaves and phloem loading of sucrose in a future article in this series). These starch-storing plastids of plants are known as amyloplasts.
The amyloplasts of plants typically express a translocator that is capable of exchanging glucose-6-phosphate for Pi on the inner chloroplast membrane (Jones et al. (2002)). This facilitates more ready metabolism to starch of the G6P derived from sucrose metabolism in the cytosol:
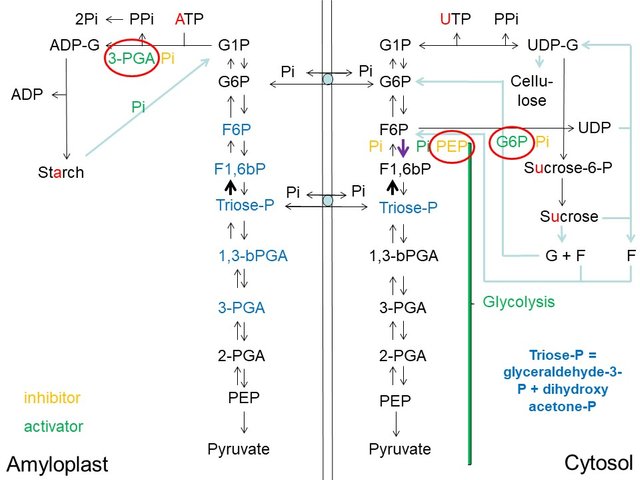
Amyloplasts Lack the Full Complement of the Calvin Cycle Enzymes and Express a Glucose-6-Phosphate/Phosphate Translocator (D. Rhodes)
Because amyloplasts do not express RuBisCO and therefore do not synthesize 3PGA by CO2 fixation, starch synthesis (via ADP-glucose pyrophosphorylase) in amyloplasts is less sensitive to fluctuations in 3PGA levels than in photosynthetic chloroplasts, and is more dependent upon relative levels of ATP, Pi and G1P.
Corn (Zea mays) (maize) accumulates large amounts of starch in the amyloplasts of the endosperm cells of its seeds/kernels. Corn starch has many uses, including as a thickening agent in food preparation, as a precursor for preparation of corn syrup (including high-fructose corn syrup), as a precursor for paper products, bioplastics, various adhesives, and for making ethanol (Corn starch- Wikipedia).
The shrunken-2 (sh2) mutant of maize lacks a functional ADP-glucose pyrophosphorylase in the amyloplast, and so is incapable of making starch. The developing ears of the shrunken-2 (sh2) maize mutant accumulate sucrose to high levels instead, and are known as "supersweet" sweet corn varieties (List of sweetcorn varieties - Wikipedia).

Sweet Corn. Image Source:
When mature, the seeds of these supersweet varieties become very shriveled (shrunken) because they lack starch. These varieties are more difficult to grow because, upon planting, sucrose in the kernels is rapidly leached from the seed to the soil, depleting seed storage reserves, and promoting the growth of bacteria and fungi. The seeds are often coated with a fungicide to limit fungal pathogen growth:
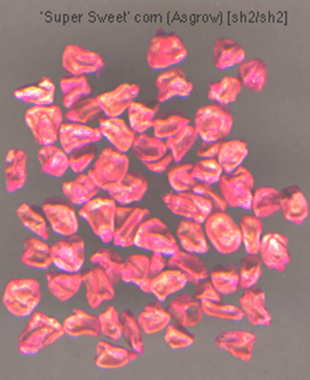
Mature sh2 sweetcorn kernels coated with fungicide (D. Rhodes)
Starch in barley endosperm amyloplasts is the major carbon source for ethanol production by yeast in the beer/ale brewing industry (see: What is the difference between ale and beer?). Starch accumulated in the amyloplasts of potato tubers is the principal source of carbon for brewing alcoholic beverages such as vodka, poitín, or akvavit (Potato - Wikipedia). During seed germination and alcoholic beverage preparation, starch is converted to maltose and glucose by enzymes called amylases (Amylase- Wikipedia).
Selected References
Additional Reading
For additional references on ADP-pyrophosphorylase in plants, see: ADP-glucose pyrophosphorylase and plant
For additional references on starch phosphorylase in plants, see: Starch phosphorylase and plant
Please feel free to ask questions or comment below. I will attempt to answer as soon as possible.
Interestingly beautiful. Your references are wonderfully placed. I have a question however. Does the starch converted into maltose and glucose by amylases in alcohol metabolize as starch when consumed in its raw form?
In alcohol production, yeast acts on the maltose and glucose under anaerobic conditions, and ferments the sugars to alcohol. This does not occur when starch is ingested by humans. I will talk more about glycolysis and lactate and ethanol production in a future post.
Thank you for clarifying me. I understand perfectly. Can't wait for the post on glycolysis and lactate production.
As I mentioned in a comment on your other post, carbohydrates are not my specialty. I read another post earlier about honey (https://steemit.com/life/@steemhealthcare/amazing-benefits-of-honey) and I have never understood why or how sugars in honey are resistant to microbial degradation, or how or why foods immersed and sealed in honey are preserved. I thought you might shed some light on it.
Good question, and I don't have a complete answer. It probably depends a lot on the phytochemicals present in the pollen and nectar that the bees collect, which in turn will depend upon the plant species from which the bee harvests the pollen and nectar. Do the bees themselves (or the microbiota of the bees?) further add antimicrobial agents to the honey to make it resistant to bacterial and fungal infection?
Certainly, honey does possess some amazing antimicrobial properties. Some honey's however, contain plant-derived alkaloids (pyrrolizidine alkaloids) that are potentially toxic to humans!
Here are a few references on this topic:
Badolato, M., Carullo, G., Cione, E., Aiello, F., Caroleo, M.C. From the hive: honey, a novel weapon against cancer. Eur. J. Med. Chem. 142: 290-299 (2017)
Denisow, B., Denisow-Pietrzyk, M. Biological and therapeutic properties of bee pollen: a review. J. Sci. Food Agric. 96: 4303-4309 (2016)
Hussain, M.B. Role of honey in topical and systemic bacterial infections. J. Altern. Complement. Med. 24: 15-24 (2018)
Letsyo, E., Jerz. G., Winterhalter, P., Dübecke, A., von der Ohe, W., von der Ohe, K., Beuerle, T. Pyrrolizidine alkaloids in floral honeys of tropical Ghana: a health risk assessment. Food Addit. Contam. Part B. Surveill. 10: 300-310 (2017)
Maddocks, S.E., Jenkins, R.E. Honey: a sweet solution to the growing problem of antimicrobial resistance? Future Microbiol. 8: 1419-1429 (2013)
Palmer-Young, E.C., Sadd, B.M., Irwin, R.E., Adler, L.S. Synergistic effects of floral phytochemicals against a bumble bee parasite. Ecol. Evol. 7:1 836-1849 (2017)
Tian, B., Moran, N.A. Genome sequence of Hafnia alvei bta3_1, a bacterium with antimicrobial properties isolated from honey bee gut. Genome Announc. 4: pii: e00439-16 (2016)
Thanks for the info! Maybe another factor is that honey would be hypertonic relative to microorganisms, changing conditions in the cytoplasm? Similar to salt preservation? Osmotic flow from cytoplasm to honey or salt.
Diluted honey (3.3% w/v) still retains antimicrobial activity against biofilms, so I suspect that it may be more than just an osmotic effect: Emineke, S., Cooper, A.J., Fouch, S., Birch, B.R., Lwaleed, B.A.. Diluted honey inhibits biofilm formation: potential application in urinary catheter management? J. Clin. Pathol. 70: 140-144 (2017)
Hmmmm. Interesting.
Excellent point!
Transfer 0.001 SBD or 0.001 STEEM to @a-0-0 to have your last blog post resteemed to over 33,700 followers. Leave Memo empty.
I don't know what to say... All these things confuse me quite a lot. It was fun looking through.
The starch produces glucose which can be found in several food. This is quite interesting looking at the breakdown process.