Insectivorous Plants - Part 4
This will be my last post in the series on Insectivorous Plants (see Part 1, Part 2, and Part 3).
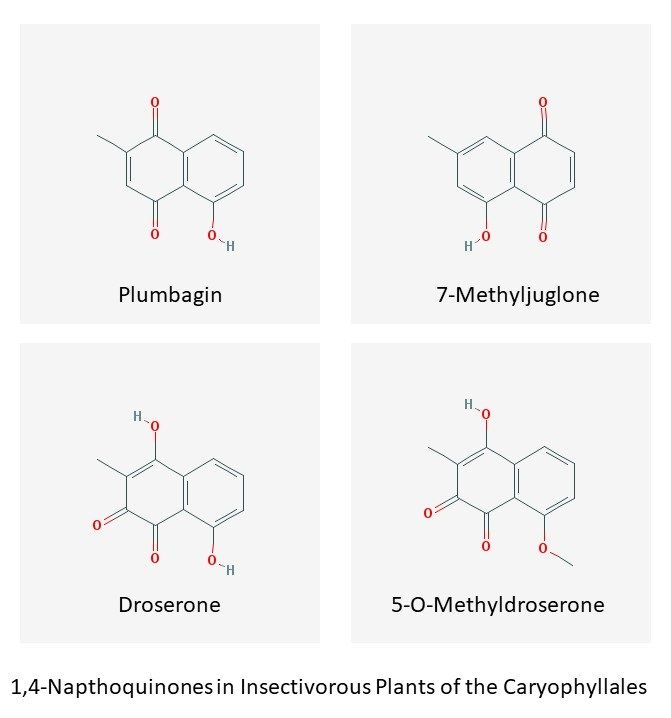
Here I would like to focus on some specialized biochemicals found in Venus flytrap, sundews and pitcher plants that may play roles in protecting the plants against herbivores and diseases, in insect digestion, and maintaining sterility of the digestive fluids secreted by the plants. These compounds belong to a class of molecules called 1,4-naphthoquinones, and include plumbagin, droserone, 5-O-methyldroserone, 7-methyljuglone and their derivatives.
1,4-Naphthoquinone structures obtained from PubChem
My colleague, Josh Widhalm and I recently published a review on 1,4-naphthoquinones (Widhalm and Rhodes (2016)). These compounds are of great interest as medicinal compounds as they have potent anti-bacterial, anti-fungal, anti-malarial, anti-inflammatory and anti-cancer activities.
Less is known about the biosynthesis pathways of these molecules than most other plant secondary metabolites, but it appears that in the order Caryophyllales (to which the Venus flytrap, sundews and pitcher plants belong) their synthesis may be via the "acetate-polymalonate" pathway. In this pathway malonyl-CoA and acetyl-CoA are condensed to form a postulated naphthalene intermediate that is then converted to either 7-methyljuglone or to plumbagin. Plumbagin is then proposed to serve as a precursor for droserone, which in turn is postulated to be converted to 5-O-methyldroserone (Widhalm and Rhodes (2016)).
Early studies by Zenk et al. (1969) on the occurrence of 7-methyljuglone and plumbagin in the Droseraceae found that they only rarely occurred together. Many species of sundews (Drosera) possessed either 7-methyljuglone or plumbagin. The snap-trap species Aldrovanda and Dionaea were reported to only contain plumbagin, and this was subsequently confirmed by Culham and Gornall (1994). More recently, Egan and van der Kooy (2012) have shown that these compounds are co-produced by Drosera species but that there is often an inverse relationship between the two. Egan and van der Kooy (2012) broadly classify Drosera species as either 7-methyljuglone-rich or plumbagin-rich. However there a few species that are intermediate and some that lack 1,4-naphthoquinones altogether (Culham and Gornall (1994)).
This appears consistent with a postulated naphthalene intermediate that can then be converted to either 7-methyljuglone or to plumbagin ((Widhalm and Rhodes (2016)). 7-Methyljuglone and plumbagin have the same molecular weight and differ only in the positions of the two =O and single -OH groups on the two rings. Plumbagin is a "regioisomer" of 7-methyljuglone (Schlauer et al. (2005)) (see figure above).
Certain plumbagin-rich species of Drosera also synthesize droserone, and in some cases 5-O-methyldroserone (via addition of a methyl group to the hydroxyl group that is not hindered by an adjacent methyl group on the ring).
The pitcher plants (Nepenthes spp.) show a similar diversity. Some accumulate plumbagin and others 7-methyljuglone (only rarely both) (Schlauer et al. (2005)). Certain Nepenthes synthesizing plumbagin may also accumulate droserone and 5-O-methyldroserone (e.g. Nepenthes khasiana (Raj et al. (2011)). Kreher et al. (1990) report that Dionaea contains both plumbagin and droserone. The production of these compounds is induced by insect-derived chitin (Raj et al. (2011)), Eilenberg et al. (2010)) and can also be stimulated by jasmonates (Krolicka et al. (2008), Ziaratnia et al. (2009), Thaweesak et al. (2011)). This is reminiscent of the chitin and jasmonate induction of digestive enzymes described in earlier posts.
These 1,4-napthoquinones can also be conjugated with various sugars and such compounds have been detected in a number of these insectivorous species (Kreher et al. (1990), Budzianowski (1996), Budzianowski (1997), Schlauer et al. (2005)). I will not discuss these sugar conjugates further, except to note that they be storage forms and prevent autotoxicity of these molecules.
The 1,4-napthoquinones are highly reactive and can generate reactive oxygen species and form adducts with DNA and proteins, and this appears to be the primary basis of their anti-microbial and insecticidal activity. Alkylation (also termed arylation) of reduced glutathione (GSH) or cysteine residues of proteins is particularly common, leading to depletion of GSH levels and/or protein cysteine chemical modifications ((Widhalm and Rhodes (2016)).
Galek et al. (1990) propose that oxidative protein modification by these compounds is a pre-digestion mechanism in Dionaea. The production of oxygen free radicals by pitcher fluid of Nepenthes has been demonstrated (Chia et al, (2004)), although it is unclear whether the sole source of these free radicals is from reactivity of 1,4-naphthoquinones.
1,4-Napthoquinones are inhibitory to the growth of food spoilage fungi and numerous other microbes (Shin et al. (2007), Gwee et al. (2014), Grevenstuk et al. (2012), Buch et al. (2012)). Buch et al. (2012) proposed that this may be a mechanism for maintaining sterility of the pitcher fluid of Nepenthes. However, more recent studies have revealed a complex microbiome within the pitcher fluid (Chou et al. (2014), Takeuchi et al. (2015), Kanokratana et al. (2016), Chan et al. (2016)). Kanokratana et al. (2016) conclude that distribution of bacterial taxa was not significantly related to the Nepenthes species but strongly correlated to the pH of the pitcher fluids (pH 1.7-6.7). But several questions remain unanswered ... how do these pitcher-associated microbes survive these often harsh conditions? In particular, do they carry enzymatic machinery to detoxify 1,4-naphthoquinones?
As noted in Part 2 of this series, the flowers of Drosera auriculata emit a blend of terpenoids (including alpha-pinene, B-pinene, and limonene) and benzenoids (benzaldehyde, benzyl alcohol and phenylethanol). The traps, however, emit linalool, geranyl acetone, B-farnesene and plumbagin (el-Sayed et al. (2016)). Notably the toxic molecule plumbagin is excluded from the flowers and is only localized in the traps. This differential accumulation of plumbagin may protect insect pollinators from injury.
Please feel free to comment or ask questions below. I will try to respond as soon as possible.
Thank you, I find the plant world to be fascinating and I am glad you are covering some interesting topics. I would like to give you a suggestion for your posts: although you cite your sources I found that some sentences are too similar to those used in the original article, this is an example:
It would be great if you could express some of these concepts with your own words. This was not meant to be a critic, I hope you take it in a constructive way ;)
I appreciate your suggestion and will strive in the future to do that. The sentence you use as an example was actually written be me in the original article, so I didn't think it would be a problem using it like that.
Thanks for the detail in your post. I've spent part of my career as a natural products organic chemist. I'm using Steemit to publish some of my unpublished data and put out ideas for projects that need to be finished.
Great. I shall definitely check out some of your posts. I'm pretty sure that I am following you. If you have any insight on some of the biochemistry that I have been talking about, please feel free to share. I'm very glad to meet someone who has interest in natural products. I've had little training in organic chemistry and I got interested in this field from the plant physiology angle.
Yes, I hope to gain followers and follow others like myself, interested in more intellectual discussions with better editorial quality than other social media platforms. I think Steemit has the potential to work as a viable alternative to the peer-review system. After all, we ARE the peer reviewers! Unlike FB, I don't have pre-existing friendships to build upon so being a newbie is much like moving to a new town and finding new friends! That's much like the life transition I'm currently in as a new retiree just having moved to Florida. Good luck and stay in touch!
I'm finding Steemit to be good mental exercise. Hope you are enjoying the warmth of Florida. It's still pretty chilly here in Indiana and I'm wondering whether we are heading for a little ice age with the approaching Grand Solar Minimum (Mörner (2015))?
Yes it is very good mental exercise, whereas FaceBook was becoming "virtual drugs" with harmful side effects.
Thanks Professor. I missed the Part 3, thus now I read both posts.
I found a related post about pitcher plants:
https://steemit.com/steemstem/@effofex/a-pitcher-plant-ecosystem-or-i-eat-flies-with-a-little-help-from-my-friends