Superoxide Dismutase - A Radical Enzyme: My Engagement to @lesshorrible's NSAS Idea Suggestion #5

This Is One Radical Idea!

In my opinion antioxidants are overhyped and radicals are misunderstood. Just like about any health myth, industry (be it big pharma or alternative pharma) took advantage of our wish for health and youth and turned a piece of the health-puzzle into a magic bullet.
Reactive Oxygen Species
A type of unstable molecule that contains oxygen and that easily reacts with other molecules in a cell. A build up of reactive oxygen species in cells may cause damage to DNA, RNA, and proteins, and may cause cell death. Reactive oxygen species are free radicals. Also called oxygen radical.
Reactive Oxygen Species (ROS) is a phrase used to describe a number of reactive molecules and free radicals derived from molecular oxygen. The production of oxygen based radicals is the bane to all aerobic species. These molecules, produced as byproducts during the mitochondrial electron transport of aerobic respiration or by oxidoreductase enzymes and metal catalyzed oxidation, have the potential to cause a number of deleterious events.
Yasuhiro Maejima, Daniela Zablocki, Junichi Sadoshima
Department of Cell Biology and Molecular Medicine, University of Medicine and Dentistry of New Jersey, New Jersey Medical School, Newark, NJ
Available online 26 June 2012 that further adds:
A primary ROS is superoxide (O2−), which is formed by one-electron reduction of molecular oxygen. Hydrogen peroxide (H2O2) is produced by reduction of O2− through dismutation. Hydroxyl radical (OH−) arises from electron exchange between O2− and H2O2 via the Harber–Weiss reaction or it is also generated by the reduction of H2O2 by the Fenton reaction.
Mutagenesis through oxidative DNA damage is widely hypothesised to be a frequent event in the normal human cell. A large body of evidence suggests important roles of oxygen free radicals in the expansion of tumour clones and the acquisition of malignant properties. In view of these facts, oxygen free radicals may be considered as an important class of carcinogens.
Ligands
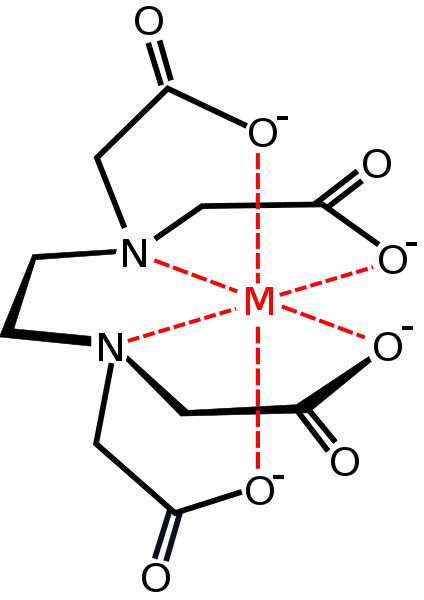
Image Author: Smokefoot, Image Source: Wikimedia Commons
Chemicool Dictionary defines a ligand as:
an ion or molecule that binds to a central metal atom to form a complex (alternatively known as a coordination entity). Ligands are usually thought of as electron donors attracted to the metal at the center of the complex. Metals are electron acceptors.
Chemistry LibreTexts goes into a little more detail about a ligand:
These complexes contain a central atom or ion, often a transition metal, and a cluster of ions or neutral molecules surrounding it. Ligands act as Lewis bases (electron pair donors), and the central atom acts as a Lewis acid (electron pair acceptor). Ligands have at least one donor atom with an electron pair used to form covalent bonds with the central atom.
Chelation is a process in which a polydentate ligand bonds to a metal ion, forming a ring. The complex produced by this process is called a chelate, and the polydentate ligand is referred to as a chelating agent.
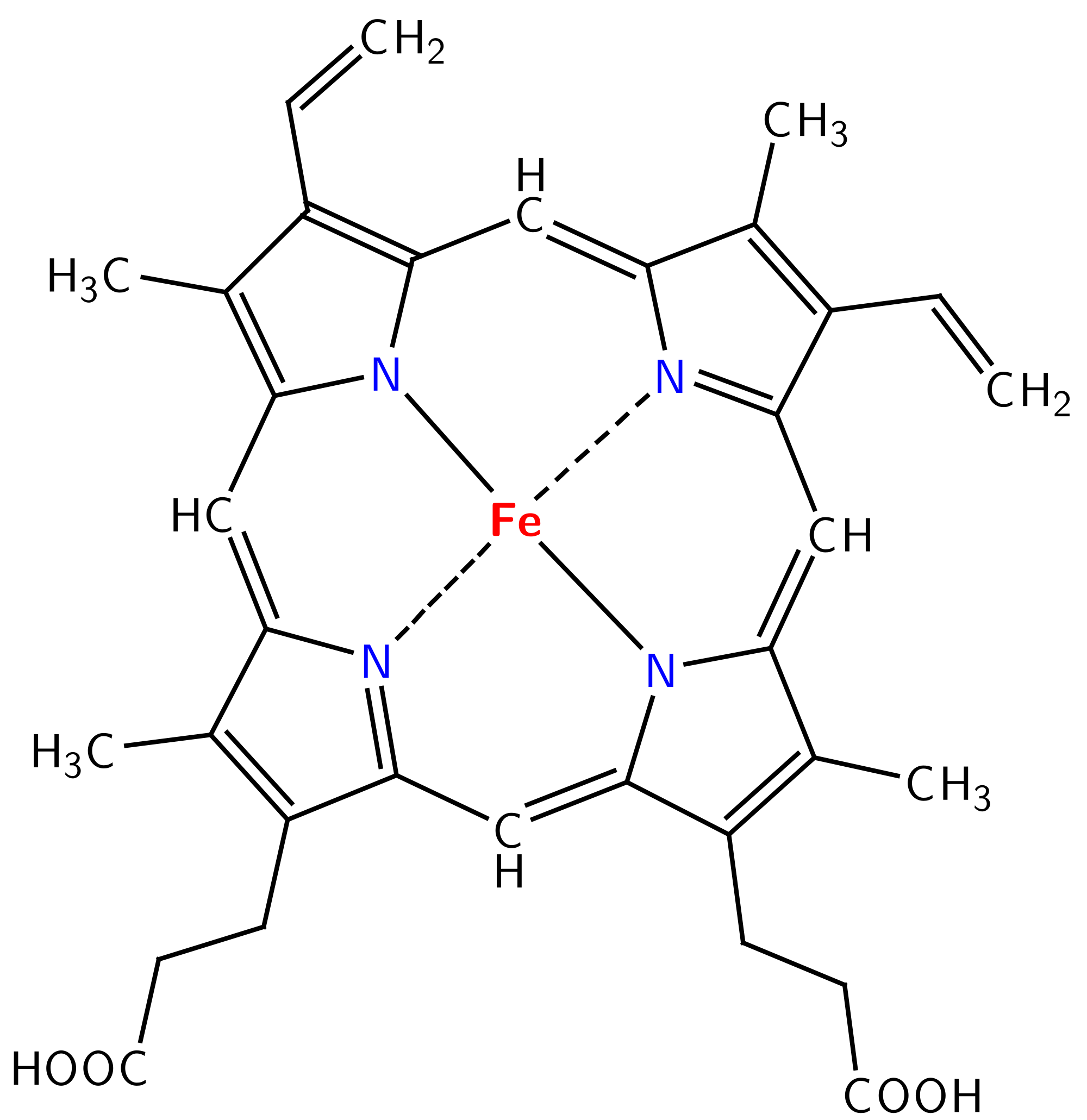
Image Author: Yikrazuul, Image Source: Wikimedia Commons
One of the most important polydentate ligands to animals in general is the Heme group used to transport oxygen throughout the body. Wikibooks describes the Heme group on its page Structural Biochemistry/Protein function/Heme group as:
Heme is a porphyrin that is coordinated with Fe(II). One of the most important classes of chelating agents in nature are the porphyrins [1]. A porphyrin molecule can coordinate to a metal using the four nitrogen atoms as electron-pair donors. In the body, the iron in the heme is coordinated to the four nitrogen atoms of the porphyrin and also to a nitrogen atom from a histidine residue, one of the amino-acid residues in hemoglobin) of the hemoglobin proteins.
Superoxide Dismutase
For years, scientists have sought a way to boost one of the body’s most powerful natural antioxidant enzymes: superoxide dismutase (SOD). Present both inside and outside cell membranes, SOD is one of the body’s primary internal anti-oxidant defenses, and plays a critical role in reducing the oxidative stress implicated in atherosclerosis and other life-threatening diseases.
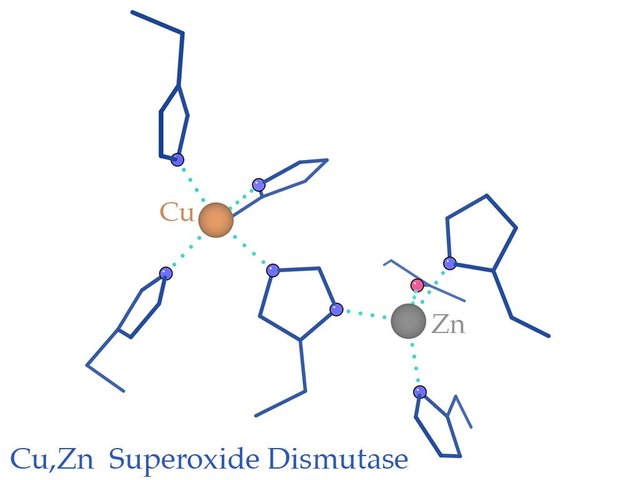
Baseline of Health Foundation further describes SOD in an article titled The Health Benefits of Superoxide Dismutase:
Superoxide dismutase (SOD) is an enzyme that facilitates the breakdown of the toxic superoxide radical into either ordinary molecular oxygen (O2) or hydrogen peroxide (H2O2). Hydrogen peroxide is also damaging, but less so than the superoxide radical, and it is also degraded by catalase. SOD works along with glutathione to neutralize reactive oxygen molecules in the body.
Superoxide dismutases (SODs) are the major antioxidant defense systems against O2•−, which consist of three isoforms of SOD in mammals: the cytoplasmic Cu/ZnSOD (SOD1), the mitochondrial MnSOD (SOD2), and the extracellular Cu/ZnSOD (SOD3), all of which require catalytic metal (Cu or Mn) for their activation.
Superoxide dismutases (SODs) have been studied for their ability to manage the oxidative state of the cell by dismuting superoxide and inhibiting signals for pancreatic cancer growth. In particular, manganese superoxide dismutase has clearly shown importance in cell cycle regulation and has been found to be abnormally low in pancreatic cancer cells as well as the surrounding stromal tissue. Likewise, extracellular superoxide dismutase expression seems to favor suppression of pancreatic cancer growth.
Inhibition of SOD causes accumulation of cellular O2- and leads to free-radical-mediated damage to mitochondrial membranes, the release of cytochrome c from mitochondria and apoptosis of the cancer cells. Our results indicate that targeting SOD may be a promising approach to the selective killing of cancer cells, and that mechanism-based combinations of SOD inhibitors with free-radical-producing agents may have clinical applications.
A large trial reported in 1994 (pdf) that daily megadoses of the antioxidant beta-carotene increased the risk of lung cancer in male smokers by 18 percent and a 1996 trial was stopped early after researchers discovered that high-dose beta-carotene and retinol, another form of vitamin A, increased lung cancer risk by 28 percent in smokers and workers exposed to asbestos. More recently, a 2011 trial involving more than 35,500 men over 50 found that large doses of vitamin E increased the risk of prostate cancer by 17 percent.
These findings suggest that when the body is given extra antioxidants, its tumor cells get to keep more of the antioxidants that they already make themselves. The cells can store the surplus, improving their ability to survive damage. This idea is supported by work that shows some genes that drive cancer growth turn on other genes that make intrinsic antioxidants.
Deinococcus radiodurans
Image Author:Michael Daly, Uniformed Services University, Bethesda, MD, USA, Image Source: Wikimedia Commons
My interest in superoxide dismutase and its ability to deal with oxygen radicals was peaked when I came across an organism known as the "worlds toughest bacterium", Deinococcus radiodurans. Genome News Network clarifies:
Deinococcus radiodurans is listed in the Guinness Book of World Records as "the world's toughest bacterium." And for good reason: The microbe can survive drought conditions, lack of nutrients, and, most important, a thousand times more radiation than a person can. The bacterium, whose name means 'strange berry that withstands radiation,' is the most radiation-resistant organism known.
Deinococcus radiodurans accumulates high levels of manganese ions, and this is believed to be correlated with the radiation resistance ability of this microorganism. However, the maintenance of manganese ion homeostasis in D. radiodurans remains to be investigated.
The protection of DNA repair and other proteins against oxidative damage is imparted by enzymatic and nonenzymatic antioxidant defense systems dominated by divalent manganese complexes. Given that oxidative stress caused by the accumulation of reactive oxygen species is associated with aging and cancer, a comprehensive outlook on D. radiodurans strategies of combating oxidative stress may open new avenues for antiaging and anticancer treatments. The study of the antioxidation protection in D. radiodurans is therefore of considerable potential interest for medicine and public health.
Conclusion
Sources Cited:


Great post! The only thing I would add is to write more in your own words, rather than just directly citing from your sources. Also thank you for using one of my ideas and including the #nsas tag. Keep up the good work!! Cheers!

That is an awesome picture you have there! I will do my best on my next post to make sure i put more in my own words and paraphrase a little more with less direct quotes. Ill will continue to engage with your #nsas, they are great ideas.
I am supposed to use this for my mentor comments:D
Awesome! I am glad that you are catching on to the #nsas ideas. And again, great work on this post! Looking forward to your next article. Cheers!
Great article. Well done!
Thanks David!! I totally forgot to touch on why I felt organometalics were electron dumps, ie pi orbitals (similar to how benzene rings have cyclic pi orbitals). But then i would have to touch on orbital systems in general and how metal ion have disassociated electron clouds.
No problem, I thought you covered the subject very thoroughly. Maybe pi orbitals would be an appropriate topic for an additional post?
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
THANK YOU Sndbox-alpha!! I try my best to produce quality content for this platform.
https://steemit.com/@a-0-0