Your Incredible Supernova Origin
It's true that we often turn to stories, based on true events or completely made up, for inspiration. A good story can make us become one with the protagonist and experience their world through their eyes, so much so that we even manage to temporarily forget where we are.

source: Wikimedia Commons
Sometimes it's nature that inspires us by making us feel tiny when looking at a majestic mountain-range, the green sea of an old jungle forest or the stars in the sky. There's a specific kind of bliss to be found in escaping the self by either transferring it to an alter ego in a good story, or severely diminishing it's size in the face of existence's grandeur. Science is seldom accused of providing anything like inspiration; on the contrary, science is often accused of the opposite. When people hear scientists explaining our deepest emotions, like love and hate, fear and happiness, in terms of electro-chemical reactions and neural connections, they feel it takes some of the "magic" away from those feelings.
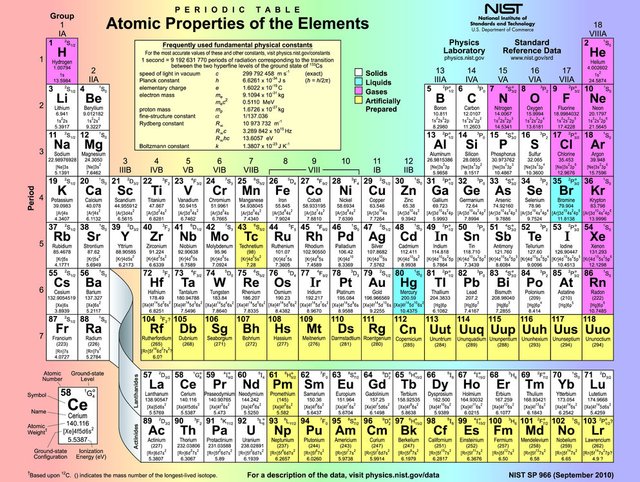
I'm going to tell the incredible scientific story of where we came from, this time by taking you back to high-school and the periodic table of the elements. This table is a miracle in itself; it doesn't just have on it the elements we know of, but all the elements that can exist in this universe. So if a UFO lands on Earth, it will be made of the stuff found on this table. We know this, because all the elements are numbered, and there are no gaps in the sequence: all numbers from 1 to 118 are accounted for. When Dmitri Mendeleev published the first recognizable periodic table in 1869, there were many gaps because a lot of the elements were yet to be discovered. And only the first 94 elements occur naturally; numbers 95 to 118 have only been synthesized in laboratories or nuclear reactors. The synthesis of elements having higher atomic numbers is currently being pursued: these elements would begin an eighth row, and theoretical work has been done to suggest possible candidates for this extension; so the table may grow yet, but still everything that exists, all stuff in the universe has a place on this table.
Now why is that, and what are those numbers? Every element is an atom. We all have heard or read sometime in our lifes that atoms are the basic building blocks of all matter. But we also know that atoms are made of even smaller parts: a positively charged nucleus and one or more negatively charged electrons. The nucleus is comprised of one or more positively charged protons and an equal number of neutrons with no charge at all. The number of each element represents the number of protons in the nucleus. So Hydrogen, number one, has one proton and therefore one neutron in it's nucleus and has one electron "circling around it." That last bit is in quotes because it's only a convenient way to visualize an atom; all these particles behave according to quantum mechanics, which is a bridge too far for this post (and my brain) ;-)
So Hydrogen is also the lightest atom. Some of the elements took a long time to be discovered because they're highly unstable or radioactive, and decay into lighter atoms within a matter of days and sometimes even less than a second; this is especially true about the elements we've created ourselves by smashing together lighter atoms to form heavier atoms. We call this nuclear fusion, and this is what powers the Sun and other active stars.
A fusion process that produces a nucleus lighter than iron-56 or nickel-62 will generally yield a net energy release. These elements have the smallest mass per nucleon and the largest binding energy per nucleon, respectively. Fusion of light elements toward these releases energy (an exothermic process), while a fusion producing nuclei heavier than these elements will result in energy retained by the resulting nucleons, and the resulting reaction is endothermic.
source: Wikipedia
source: Wikimedia Commons
So now you know why active stars that are still shining can only make elements up until iron and occasionally nickel; fusion of light elements towards these not too heavy elements releases the energy we see as light. Our Sun isn't a very big star though and that's why it isn't even able to form iron, or any elements higher than number 8 on the periodic table, Oxygen. Why is this important? That'll become clear soon enough, dear reader, but for now I'd like you to consider what you're made of. I think it'll come as a surprise to no one when I say we're all made of water mainly. Around 65 percent of the mass of our bodies is plain old water, infants are even 75 percent water. A water molecule is made up of two Hydrogen atoms and one Oxygen atom, which would lead us to believe, on the surface, that we're mainly made of that element, and this is true if we count atoms, but not is we measure mass or weight. One Oxygen atom has 8 protons, neutrons and electrons, and two Hydrogen atoms only have 2 of each; so most of our mass is Oxygen and most of our atoms are Hydrogen. Forget "you are what you eat," because you clearly are what you breathe and drink. It must be said though that Hydrogen only scores third place in the mass-contest because of it's extremely low atomic weight; number two on that ladder is Carbon.
Right after the Big Bang only sub-atomic particles existed; protons, neutrons and electrons were the first and only inhabitants of the fledgling universe. Conditions were so hot that in the first couple of minutes the lightest atoms were formed by some fusion process. There was only enough time though to fuse together Hydrogen, Helium and a tiny bit of Lithium; the universe expanded and cooled off so fast that after only three minutes conditions were too cold for this "spontaneous" fusion to continue. There also must have been a slight imbalance, it is theorized, in these first moments of the universe's existence, between the amounts of matter and anti-matter, or else nothing would exist at all (they annihilate each other), and there also must have been, very early on, a not completely uniform distribution of these primordial particles for the clustering of matter we see now to have evolved.
Three minutes after the Big Bang, only the first three positions on the periodic table were filled. And Hydrogen was not only the first atom to ever exist, it's also by far the most commonly found atom in the entire universe; 75% of the atoms in the universe are Hydrogen, 24% is Helium and the rest is just 1%. But if we finish the top 10 we get:
- Hydrogen
- Helium
- Oxygen
- Carbon
- Neon
- Nitrogen
- Magnesium
- Silicon
- Iron
- Sulfur
Keep that in mind, but realize right now that the stuff you're made of is essentially as old as the universe itself; the protons, neutrons and electrons that make up all of your atoms were created 13.7 billion years ago. These elementary particles fused together in the first three minutes after the Big Bang to form the first three elements on the periodic table in a rapidly expanding and cooling universe. As an apropos, Helium is a noble gas, which are all neatly grouped in the right-most column of the table. This Hydrogen and Helium gas over time started to drift and clump together under the influence of gravity, and as some of these clumps of matter grew big enough, temperatures and pressures in their centers became high enough for the fusion process to start again. This time Hydrogen was fused to form Helium. Then Helium could fuse with Helium of Hydrogen, forming even heavier atoms, and so on. All this fusion needs higher pressures and / or temperatures as the atoms get heavier; this is why a modestly sized star like our Sun will never be able to make Iron, nor will it ever release the atoms it does make back into the universe...

source: Wikipedia
Only the biggest stars end their life with a grand explosion and go supernova. These massive stars can take the fusion process all the way op to Iron. The heaviest elements are made only in the very center of such a star and thus it's nucleus is an ever growing Iron ball. This can't go on indefinitely because gravity will cause this Iron ball to collapse in on itself after a certain threshold is reached. This collapse takes only a second or two and causes such high pressures and temperatures during those few seconds that the heavier atoms can be made, up until the atomic number 94 for the biggest of them all. This most violent of explosions can briefly outshine an entire galaxy, such are the forces involved, and it is the violent death of such gigantic stars we owe our existence to, as they are the ones "kind enough" to release their contents with all the building blocks of planets and organic life as we know it, forming an immense cloud, often several light years across. The heavier elements, like gold, are the rarest elements in the universe because stars can only produce the during these few seconds at the very end of their lifes...
Around 4.5 billion years ago our solar system was formed from the gravitational collapse of such a cloud, often called a molecular cloud or Stellar Nursery. "Our" nursery probably birthed several stars, and around one of them the matter clumped together to form the Sun, planets, moons and asteroids, and our Earth was among them. The story from here on is relatively short: after just one billion year of the Earth's existence somehow the stuff from our stellar nursery came together to form the first living cells. On a higher level, these cells are the building blocks for more complex lifeforms, all of which are heavily dependent on the universe's most abundant atoms, two of which combine to make the water we're mostly made of and upon which all life depends; it's called the life giver for a good reason. The most miraculous thing in existence is you, my friend. Matter has taken 13.7 billion years to come together in you, for the briefest of of times, to be able to reflect upon itself. And the stuff you're made of has traveled through the lifetimes of several stars that exploded with a force and brightness that can not be described in words. This very moment you're literally stardust reflecting on previous lifes ;-)
And the kicker is that you'll end up as stardust, and will most definitely become part of some star, or stars, in the future, starting with the Sun. Our local star is not that big, it won't explode. But as the process of nuclear fusion continues, the temperatures and pressures in it's core will rise, causing the outer layers to expand away from that core, becoming a red giant so big it'll engulf the Earth, before collapsing into a white dwarf. Don't worry though; the Sun still has enough Hydrogen to burn for the next 5 billion years. But by then, our stuff will be re-united with the stars... Ashes to ashes, stardust to stardust. If this 14 billion year saga of where you came from, and where you're going doesn't make you realize in a profound way how special you are, how much of a miracle existence is, how lucky we are to experience anything at all, I don't know what to say. And there's also so much more to say about this, but I'll have to leave that to you, dear reader, as I already have overstayed my welcome; sorry for getting carried away a bit, and you have my sincere gratitude for staying this long. Remember it's only a minuscule fraction of an instant on the timescale we've just covered ;-)
What Is A Supernova?
Thanks so much for visiting my blog and reading my posts dear reader, I appreciate that a lot :-) If you like my content, please consider leaving a comment, upvote or resteem. I'll be back here tomorrow and sincerely hope you'll join me. Until then, keep steeming!

Recent articles you might be interested in:
| Latest article >>>>>>>>>>> | Arcane Arms Race? |
|---|---|
| Rich In 3 Simple Steps! | Rich Debt, Poor Debt |
| Paranoimia | Break The Matrix |
| Forest Through The Trees | Cheaper Than Slaves |

Thanks for stopping by and reading. If you really liked this content, if you disagree (or if you do agree), please leave a comment. Of course, upvotes, follows, resteems are all greatly appreciated, but nothing brings me and you more growth than sharing our ideas. It's what Steemit is made for!


Just for Full Disclosure, I'm invested in these crypto-currencies:
Bitcoin | Litecoin | EOS | OmiseGo | FunFair | KIN | Pillar | DENT | Polymath | XDCE | 0x | Decred | Ethereum | Carmel | XYO

@helpie is a WITNESS now! So please help @helpie help you by voting for us here!

Good one!
captain.palnet will follow :)
Thanks so much @captain.palnet! :-)
I believe the universe is a big living entity!
Posted using Partiko Android
I think you may well be right my friend :-) Although it has to be said: if the universe is one giant consciousness, as many believe, than the advent of Fiat money must have been the first sign of cosmic Alzheimer.... ;-)
The universe might be right on crypto. ;)
Hi @zyx066!
Your post was upvoted by @steem-ua, new Steem dApp, using UserAuthority for algorithmic post curation!
Your UA account score is currently 3.506 which ranks you at #6083 across all Steem accounts.
Your rank has dropped 5 places in the last three days (old rank 6078).
In our last Algorithmic Curation Round, consisting of 250 contributions, your post is ranked at #172.
Evaluation of your UA score:
Feel free to join our @steem-ua Discord server