The Science of DNA Extraction, and the use of Sodium
The human cell structure is composed of countless deoxyribonucleic acid (DNA) molecules. DNA which is found within a cell’s nucleus is a molecule containing information about the body’s genetics.
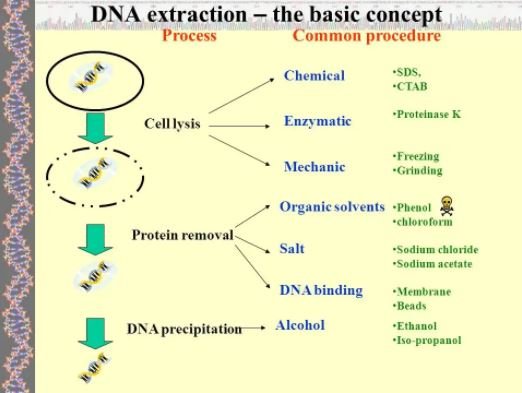
The procedure for extracting DNA comprises of series of steps which require the gently breaking up of the cell, the nuclear membrane, and the separation of the DNA from proteins and causing it to precipitate in a solution.
Accomplishing this process of DNA extraction involves using different chemicals according to the structure of the membranes, the deoxyribonucleic acid and the electronegativity of the DNA.
Compounds of sodium such as sodium chloride are used after the DNA has been stripped off its proteins, to stabilize the DNA. The sodium compound also aids in the DNA precipitation.
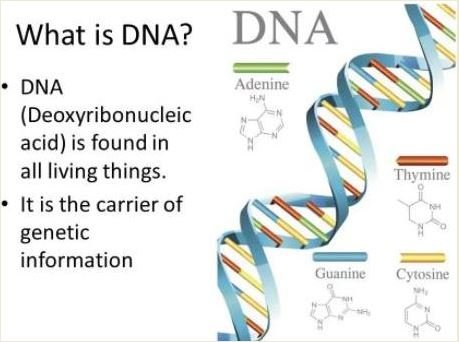
The DNA Structure
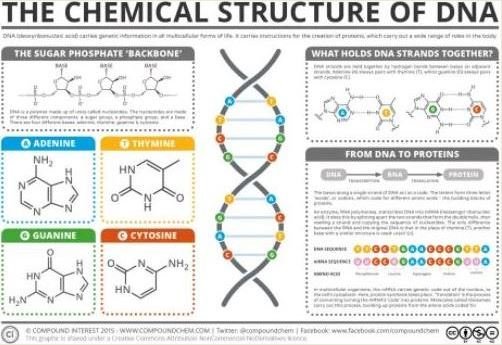
Two long strands of nucleotides which are surrounded with sugar-phosphate backbones make up the structure of DNA. Further arrangement of the DNA involves the twisting and wrapping itself with associated proteins in order to stay organized and strung together.
The sugar-phosphate backbone is the mostly closely exposed portion of the DNA to the environment, in the native state. The environment within a cell is mostly water and in it, DNA is soluble due to its general polarity.
The Polarity of DNA
In chemistry, when molecules are found to contain an unequal distribution of electrical charges, they are described as having “polarity”.
The nucleic acids are polar in nature, according to Paul Zumbo of the Cornell Medical College. In the DNA, the phosphate groups which are polar on the backbone carry more negative bags, and this accounts for solubility of the water because water is polar, as well.
The negative charges if DNA interact with the positive charges of water and then create a solution. Therefore, in order to extract the DNA for testing, the DNA would be precipitated out of the water solution.
Ideally, water has a relatively weak positive charge, and to precipitate the DNA out of the solution of water, a stronger positively charge ion is provided in the solution. This is where sodium comes into use.
Alcohol and Sodium for precipitation of DNA
After extracting the DNA from the cell’s nucleus and precipitated in the water, sodium ions are introduced and they create a temporary bond between the sodium and the backbone. This causes the DNA to become temporarily neutralized, and then it can be separated from the water solution.
The next stage is the introduction of alcohol such as ethanol or isopropyl alcohol, which forces the DNA to be tightly bonded, because alcohol is non-polar in nature. As soon as the DNA is separated from the solution and tightly bound to the sodium, it can be precipitated into concentrated form out of the solution, for the aim of purification or to be visually analysed using a smooth glass rod and spooling it.
Some other steps in the extraction of DNA
A detergent is often used to break up the molecules of lipids. This is done before breaking down the plasma and nuclear membrane in order to access DNA from cells.
The common detergent used in the laboratory from the procedure is SDS (sodium dodecyl sulfate). However, when it comes to simple extractions, ordinary soap within reach can suffice.
Reference: r1, r2, r3, r4, r5, r6, r7
Thank you for your time and for reading my post.
If you found this post interesting, then kindly UPVOTE, RESTEEM and FOLLOW @rickie, for more quality posts.
You Can Check Out My Other Posts Below:
- The future of wireless communication hinges on Terahertz frequencies
- [Sansanmycin Uridylpeptide]: The New compound from Soil Bacteria to eradicate Tuberculosis
- Monitoring Diabetes Just Got A Little Bit Easier With Nanotechnology
- The Future of Space Food for Astronauts Could be Human Excrement (Faeces)
- A Bacteria Has Been Found To Produce Gold By Consuming Metals
- The future of Oil-Spill Cleanup could be Oil-absorbing Cellulose

Great work. Have good day! Thanks you for sharing
Interesting piece. So this is what goes on when someone goes for a DANA test? Science is very intriguing. Wow. Thanks @rickie for sharing.
Yea, it is @zizymena. Thanks for visiting.