How do we know the shape of a molecule? Chemistry with Battlr Ep. 4
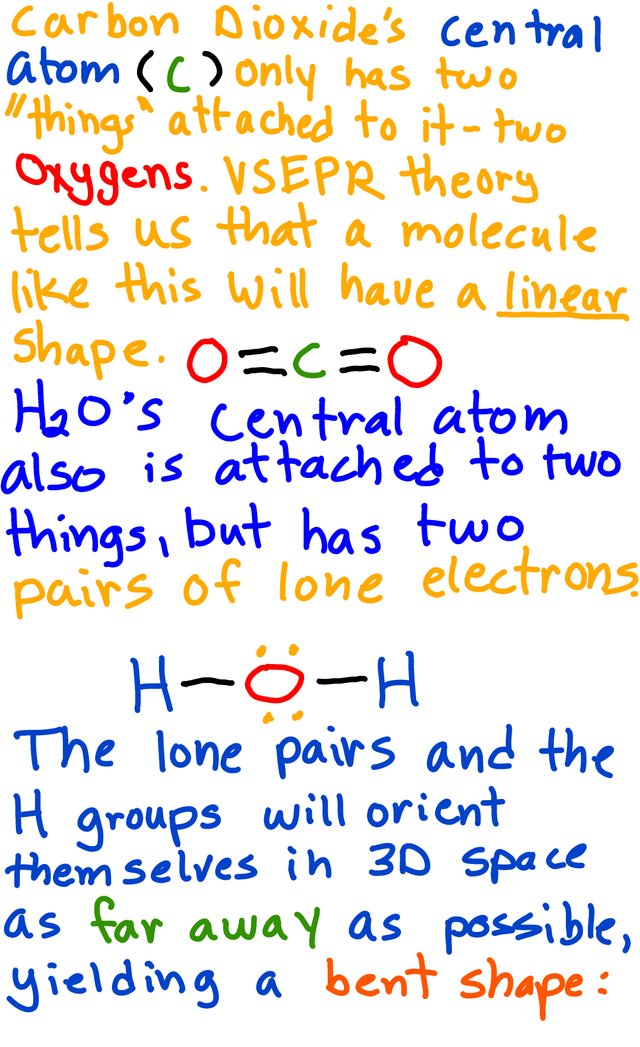
Here is an example of what a linear molecule looks like in 3D:
Bent:
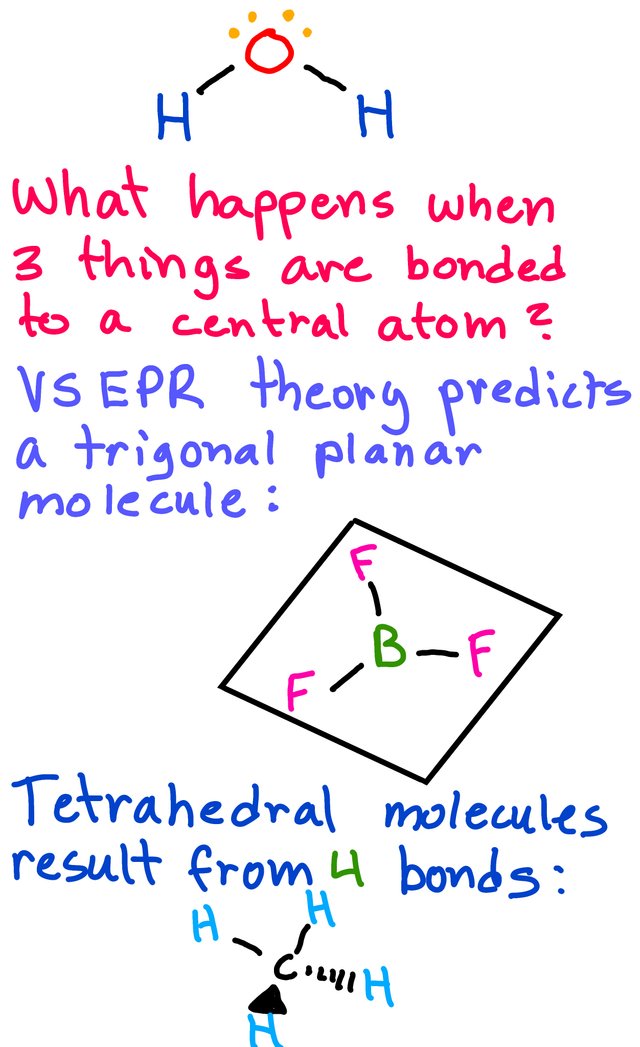
Trigonal Planar:
Tetrahedral: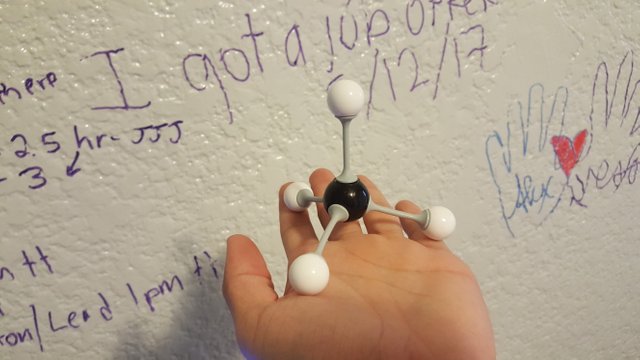

| Past Episodes | |
|---|---|
| Ep. 1 Atoms and Molecules | https://steemit.com/science/@battlr/what-is-the-difference-between-atoms-and-molecules-battlr-s-chemistry-series-ep-1 |
| Ep. 2 What are Ions? | https://steemit.com/science/@battlr/what-are-ions-chemistry-with-battlr-ep-2 |
| Ep. 3 What is Electroplating? | https://steemit.com/science/@battlr/what-is-electroplating-chemistry-with-battlr-ep-3 |



I know this is an extremely simplified explanation of VSEPR theory, so questions are welcome! If you want to learn more from videos, here are a couple great sources on VSEPR theory:
VSEPRT is problematic and some how get stuck as you go in to more detail analysis. One of the classic examples would be the inequivalent of the lone pair on oxygen of water molecule. Take a look here if your are interested :D
But I still think VSEPRT would be a good start for learning chemistry at the very beginning
Actually with the current technology, we can really visualize molecule by atomic force microscopy(AFM)
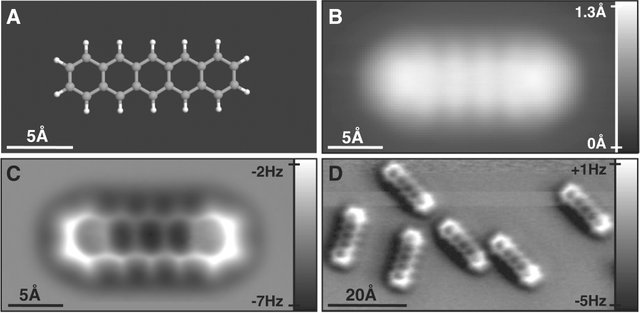
Here is an example of pentacene nicely demomostrated by IBM with AFM
Frankly, I dont know much about this technology so may be see if some expert can explain a bit lol.
Did you read the article you linked? The article was against retiring the VSEPR model:
"Critical analysis, which includes a detailed examination of the photoelectron spectrum of methane, reveals these premises (premises on which the anti VSEPR advocates established) to be ill founded and inconsistent with modern electronic structure analyses. Placed in a modern context, the hybrid orbital concept helps to familiarize students with the methods of working chemists, foster construction of a deeper, more interconnected understanding of chemistry and its connection to the laws of nature, and provides a secure foundation for more advanced chemistry classes."
I do agree that VSEPR is a good model to be used for into chemistry classes. There are even exceptions to some molecule structures within VSEPR theory itself, for example The gas phase structures of the triatomic halides, AX2E2 molecules which are linear rather than bent, and AX6E1 molecules, which are octahedra rather than pentagonal pyramids. So the theory does recognize that there are exceptions to the rules. I wouldn't say that just because in advanced and specific cases that the theory should be done away with!
My university has an AFM lab! But it is only open to graduate students and really only works with very specific cases.
Oh! I am terribly sorry that I mistakenly attached the old publication by F. Weinhold in 2012, as he made a few comments on VSEPRT.
The one I was originally trying to show you was this one which is done by Weinhold in 2014.
"VSEPR-style representations of orbital shape and size are shown to be fundamentally inconsistent with numerous lines of experimental and theoretical evidence, including quantum mechanical ‘‘symmetry’’ principles that are sometimes invoked in their defense. VSEPR-style conceptions thereby detract from more accurate introductory-level teaching of orbital hybridization and bonding principles, while also requiring wasteful ‘‘unlearning’’ as the student progresses to higher levels"
This publication was made in to a lecture material in the Harvard University which I originally came across in the online teaching materials
Ah! Will do! I didn't know this was a tag!
The quality is rapidly improving, I must say. Combining this with a concise video I think would be your best bet time allowing for the full feature package.
It makes so SO happy to hear this. I really have put more effort into formatting and presentation, and I am so glad that you noticed. Thank you so much for your encouragement! I definitely would want to invest in some good lighting/setup for videos. Thank you so much!
I wonder how it would look if you tried it agaisnt a black background.....like colored chalk on a blackboard khan academy type look. I think it would help certain colors particularly yellow and overall be a bit easier on the eyes. Yellow is a pretty annoying color to use on a white background - https://www.sitepoint.com/10-troublesome-colors-to-avoid-in-your-advertising/ Then again it does say colors are hard to read agaisnt black background, so I'm not sure maybe that is a bad suggestion.
You should also talk with an animator at some point when you decide to try videos, I think that would work quite well.
Woohoo! Followed