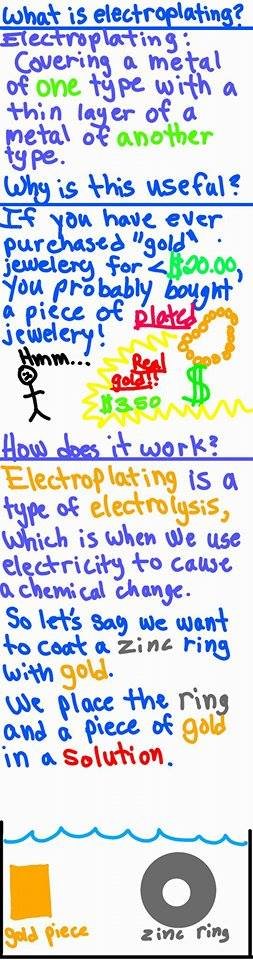
What is electroplating? Chemistry With Battlr Ep. 3



7 years ago in #science by battlr (53)
$7.99
- Past Payouts $7.99, 0.00 TRX
- - Author $7.12, 0.00 TRX
- - Curators $0.87, 0.00 TRX
oh cool! So they use real gold sheets to electroplate? does it decrease the mass of the gold plate over time?
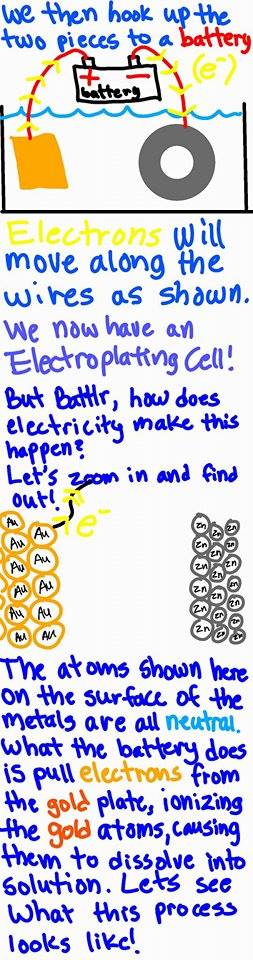
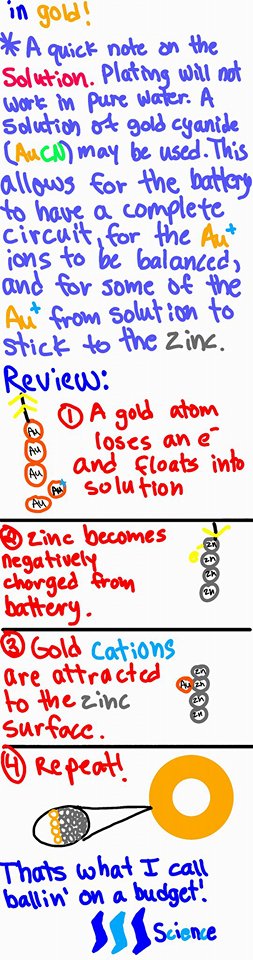
Yes and yes! Some textbooks will show the gold material that is being used for plating shrink over the course of the reaction in order to show that the gold material is indeed being used to coat the material.
In a class that I took where we plated using copper, we weighed both the copper and material to be plated before and after the reaction. We found that the amount of mass that the copper lost was = to the amount of mass that the material gained!
That is super cool! It's hard to believe ancient people used to electroplate in Iraq.
Thanks for the info!
Definitely is hard to believe, and this article does say that this is only a hypothesis, but they definitely were on to something!
Glad you enjoyed the post!
True, on the hypothesis.
It;'s really difficult trying to explain how a culture from 2000 years ago understood the principles of building and using a battery. :)
I had no idea this is how it's done. So educative! Thanks.
Im so glad that you learned something! This is one of my favorite subjects in chemistry!
Is this how metal alloys are made as well?
Not quite. Metal alloys are made by heating two metals to their melting point and then mixing them. After they cool, you will get an alloy of the two metals. For example, if you mix copper and zinc, you get a brass alloy. Steel is an alloy of iron and primarily carbon.
Congratulations @battlr! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP