Three-Process Thermodynamic Cycles with Review Problem (English Units) | Part 1
Hi folks!
Contrary to what I have previously shared about Otto and Diesel cycles wherein you can notice that it is made up of 4 thermodynamic processes, well today, allow me to share about review problems (this will be the first part out of 2, since I have in here two problems not identical wherein both are expressed in different units of measurement, English and SI units to be exact) wherein 3-process cycles are being showcased.
So without so much ado, let me introduce this review problem which tackles 3-process cycle.
Two and one-half pounds of airactuate a cycle composed of the following processes: polytropic compression 1-2 withn = 1.5; constant pressure 2-3; constant volume 3-1. The known data are: p1 =20 psia, t1 =100 degree Fahrenheitand QR =-1682 BTU. Determine the following:
- Temperature at the end of isentropic compression or at the beginning of constant pressure process (T2) and the temperature at the end of constant pressure process or at the beginning of constant volume process (T3),
- The Work (WN) of the cycle using the Pressure vs. Volume (PV) plane,
- Heat Added to the cycle (QA),
- Thermal efficiency (e), and
- Mean Effective Pressure (PME)
In the above review problem, the key to solving it is thru mastering either the Pressure vs. Volume (PV) Diagram of each of the five thermodynamic processes and in that way once you connect and plot all the processes especially the ones being mentioned in a three-process cycle you’ll be able to obtain the needed parameters and analyze its ideal performance since you have obtain the concept first and the calculations will just follow and flow right after. And the creation of a Pressure versus Volume (PV) diagram will be our first step in solving this problem and the drawn PV diagram is shown below.
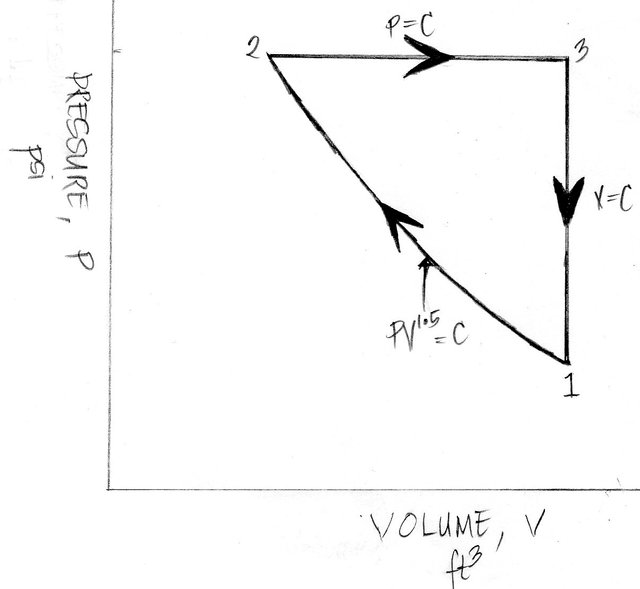
So let me start showing how I made my calculations for the above review problem.
Temperature at the end of isentropic compressionortemperature at the beginning of constant pressure process(T2) and thetemperature at the end of constant pressure processortemperature at the beginning of constant volume process(T3)
In solving this portion of what is being asked on the review problem, the first to obtain as for me is to obtain the temperature at the end of the constant pressure process and it can be easily provided with the amount of energy being rejected by the 3-process cycle. And the computation goes like this:
The negative in the value of heat rejected signifies that amount of heat is rejected and additionally being done “by” the cycle. And with that we are able to obtain a
4485.32 degree Rankinefor the temperature at the end of constant pressure process or the temperature at the beginning of isometric process.
The next thing to do before we obtain thetemperature at the beginning of isobaric process(T2) is to obtain first thepressure at the end of the isobaric process(P3) and you might be wondering why thepressure at the beginning of the isometric process(P3) is obtain first. The very reason for that is P2 is just the same to P3 since they operate in isobaric process and it will be very helpful in computing for the temperature at the end of the polytropic compression (T2). And the computation goes like this:
The pressure during the isobaric process portion of the 3-process cycle is equal to
160.19 psia,(P2 = P3).
Finally, we can now solve for thetemperature at the end of polytropic compression(T2) and the computation goes like this:
Thus the temperature at the end of polytropic compression is equal to
1120.44 degree Rankine.
- The
Work(WN) of the cycle using the Pressure vs. Volume (PV) plane,
As for this portion of the review problem, there is no shortcut to this one but the work can be obtain sincework done in the cycle is equal to the summation of each work being done per processwhich is shown in the following formula.
And using the above general formula, we have an equation for the work of the 3-process cycle as:
Wherein:
So let’s start computing for the work done for the Process 12 wherein it is a polytropic compression for which n = 1.5. And the computation goes like this:
The work done during the polytropic process is equal to
-192.12 BTUnegative indicates that it is donebythe cycle.
As for the work done during isobaric process, the computation goes like this:
The work done during the isobaric process is equal to
576.74 BTU.
And as for the work done during isometric process, it is understood that is equal to zero since the volume is constant as shown in the formula below which is derived by way of integration.
Finally the summation of all the being done by each process of a 3-process cycle is equal to the total work done and the computation goes like this:
The work done by the 3-process cycle is equal to
384.62 BTU.
Heat Added to the cycle(QA),
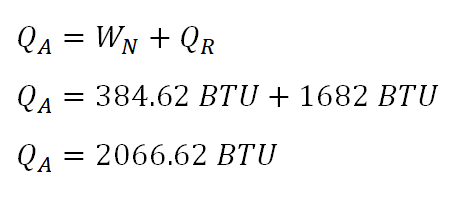
As for the principle in obtaining the heat added of the 3-process cycle, it can be directly obtain by adding bothwork done by the 3-process cycleandheat rejected by the 3-process cycle(the absolute value only, since thatnegative signindicates that it is rejected by the system or in other words donebythe system.). And the computation goes like this:
The heat being added to the 3-process cycle is equal to
2066.62 BTU.
Thermal efficiency(e), and
As for the thermal efficiency, the computation goes like this:
The ideal thermal efficiency is equal to
18.61%.
Mean Effective Pressure(PME)
In obtaining for the mean effective pressure of this 3-process cycle, we need to check first the PV diagram and check what are the largest and smallest volumes being experienced all throughout the cycle and based on the PV diagram it is both V1 and V2. And since it is not provided in the problem, we need to obtain those two important parameters. And let’s start with the V1 which is the volume at the beginning of polytropic compression. And the computation goes like this wherein it uses the universal gas constant formula,PV = mRT:
And after the computation it was found out that the volume at the beginning of polytropic compression is equal to
25.93 cubic foot.
And as for the volume at the end of polytropic compression, it can be obtain using the polytropic relations of pressure and volume. And the computation goes like this:
The volume at the end of polytropic compression is equal to
6.48 cubic foot.
Finally, we can now proceed to the calculation of the mean effective pressure of the 3-process cycle. And the calculation goes like this:
The mean effective pressure of the 3-process cycle is equal to
106.84 pound force per square inch (psi).
And that was some sort of calculations that is a bit small in quantity as compared from my previous blogs relating to thermodynamic processes and cycles for internal combustion engines. And this sums up my blog post about 3-process cycle wherein I have pulled up a review problem wherein it is expressed in English units.
Thank you for spending your time reading this blog of mine and I do hope you have learn something out from this blog, especially to mechanical engineering students.
Much love and respect.
Ace | @josephace135
Reference:
Hipolito B. Sta. Maria, Thermodynamics
Solutions are being made by me and the sample review problem is found in page 92, problem number 4.
The above PV diagram for this review problem was manually made by me in preparation for this article.
Presentation of equations and formulas were made possible by MS Word 2010 Equations function.
Screenshots were made possible by utilization of Snipping Tool application.
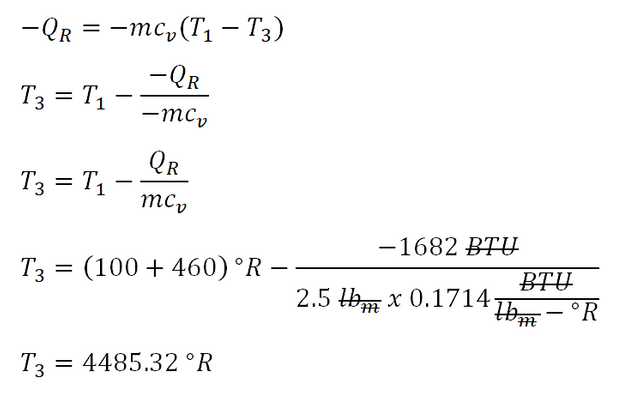
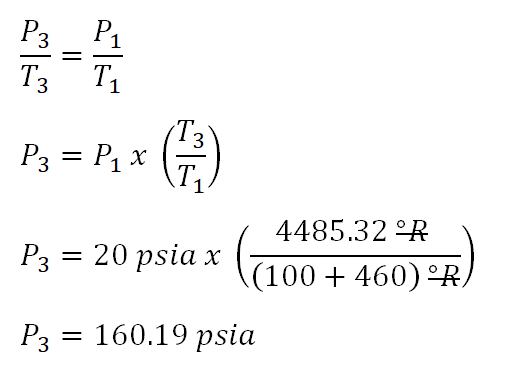
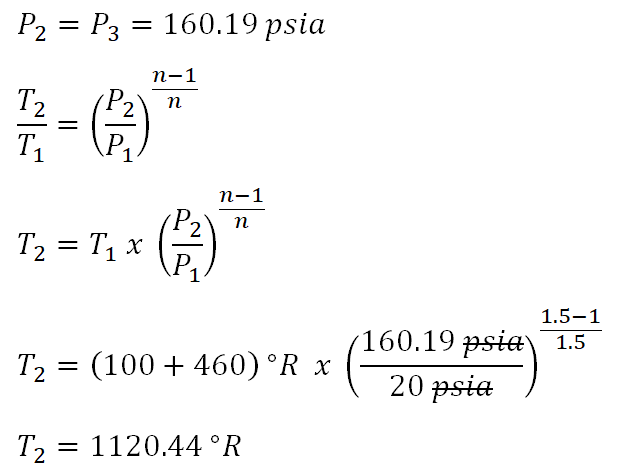
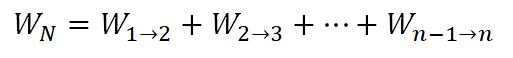
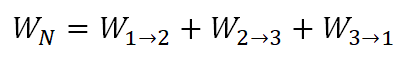

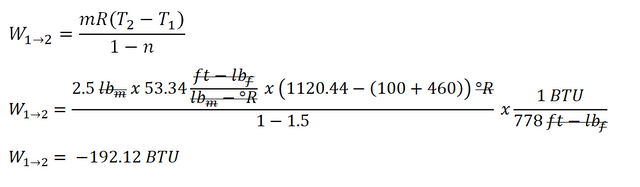
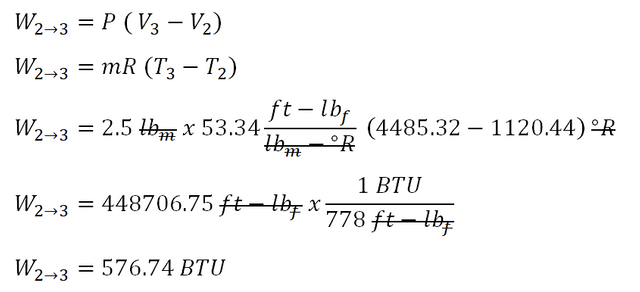
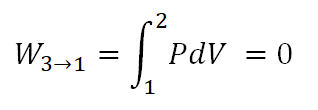
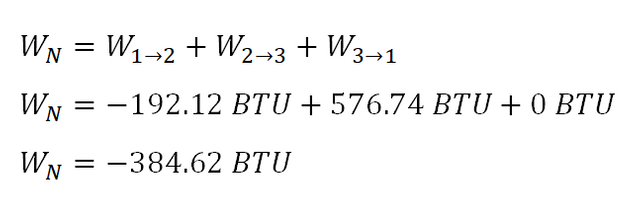
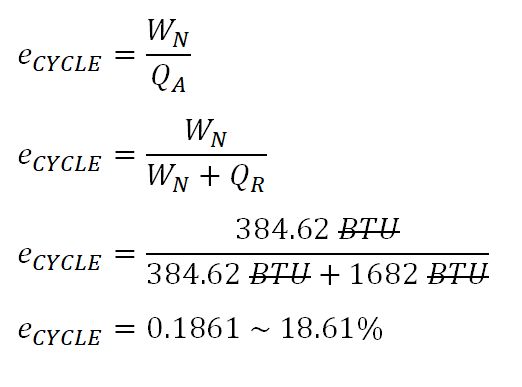
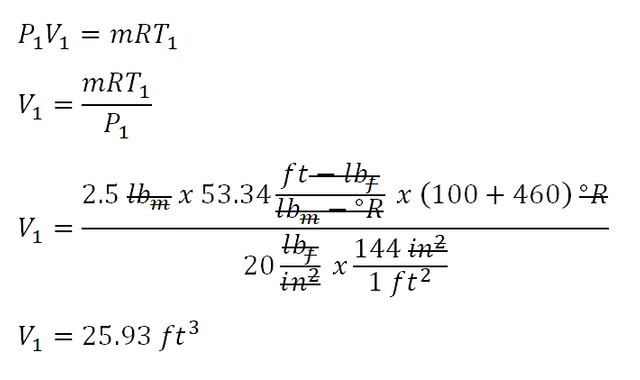
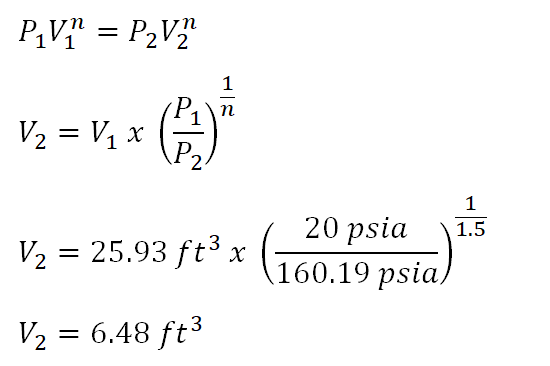
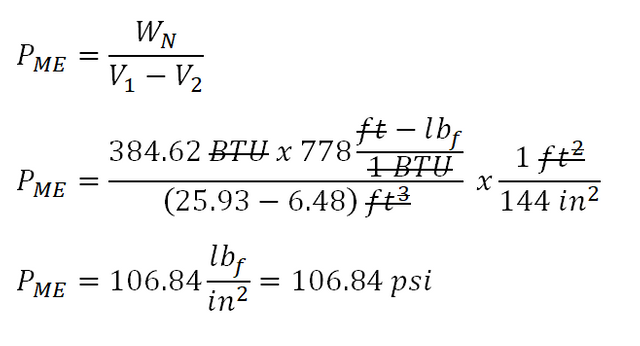
Your blog has received an upvote from the communal account of Steemph.antipolo for being an active discord member and as an active community member. Keep up the good work and best of regards. Keep on Steeming!
You can get a support by joining our discord channel and gain votes from
our curators. Join our discord now
https://discord.gg/7w3hJqw
If you would like to support steemph.antipolo project you can help by delegating your spare SP to us, just click the link below.
50 SP 100 SP 200 SP 300 SP 400 SP 500 SP 1000 SP
Good work! Thanks for sharing this education. I will follow this serie.
Thank you bro @sargoon. Likewise I’ll stay updated on your tutorial series.
Hi, I just read some of your thermodynamic related post and I fascinated by what you have write. Hope next mathematical model will be based on real device/hardware. (or something that can be controlled using electricity)
Hi @drsensor, thank you for passing by. Hopefully in the foreseeable future I’d be able to pull up some calculations being applied to real device as you’ve mentioned.
This is interesting, I went through business in high school so I've never came across this in my life. Only heard of it in movies, its good to see the model and calculations being explained
Thank you @alvinauh for passing by. Btw, in Philippines this is being taught in college especially mech engg students.
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @bitrocker2020, @zord189, @aaronleang, & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.