Does This Animal Produce Gunpowder ?
Nitrate
Nitrate
Hello Steemian,
About four days ago, i feel unwell, but my spirit was back to write science article in steemit, okey pass week ago i told about Potassium Nitrate history and how they use potassium nitrate in ancient times, now i will continue to write how we can find potassium nitrate from natural material.
Goat is mammals belong to herbivorous animals and also chew the breed. Goats have the same body size as the Sheep. Mutton can be eaten. Goat milk can also be drunk and also used as cosmetic ingredients. Goat urine has enormous benefits to overcome problem of farmers' dependence to use of chemical fertilizers (inorganic), agricultural land various types of chemicals either from fertilizers, pesticides, herbicides and fungicides. Goat urine (livestock waste) compared to cow urine is slightly superior, this is because the chemical content contained in the urine of goats has been known more content as H2O, Ca, Cl2, K, Na, Mg, N, N, Nh3, So4 and PO4, urea, creatinine, uric acid, Urine beneficial ingredients can be separated from waste, and used to make fertilizers, medicines, brain cells and gunpowder.
Potassium Nitrate
Potassium Nitrate
Gunpowder consists of 75% potassium nitrate, 15% charcoal and 10% sulfur. While charcoal and sulfur have been relatively easy to obtain, potassium nitrate is not commonly found in nature. Early sources are found in caves where guano been combined with minerals from cave walls, Soaking and filtering guano is an effective method, and there are so many caves, and so many bats. The solubility of potassium nitrate in water is high enough, in 0 C and 1 L water, the sap can reach 133 gr. This amount of solubility is quite massive although not as much Sodium Nitrate that can dissolve more at the same temperature and volume.
Physic Fact
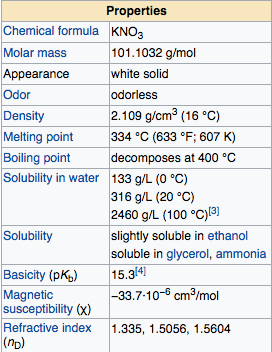
Potassium Nitrate is a solid salt compound in room temperature, this compound is white crystalline and odorless metallic crystal. The solubility of potassium nitrate in water is good enough, at O C potassium nitrates soluble at 133 g/l, temperature 20 C of soluble potassium nitrate 316 g/l, and at temperature of 100 C of soluble potassium nitrate of 2460 g/L. If we observed, the solubility of potassium nitrate is higher along with the increase in water temperature, When dissolved into water, this compound will absorb energy of environment or reaction is endothermic solvents , so that water temperature will drop when we dissolve this compound. The pH of the KNO3 solution ranges from 6.2 to 7.0, indicating that the KNO3 compound is neutral. Not as NaOH compounds, the compound of potassium nitrate is not hygroscopic.
Chemistry Fact
Potassium Nitrate is a powerful electrolyte compound, when dissolved into water, potassium nitrate ionizes into K + and NO3- ions. In addition to showing ionic compounds, potassium nitrate is also a "Oxidizer" or the strong oxidizer agent. This means it can oxidize other substances, while it itself undergoes reduction. When its react with a compound that reduce the reaction can cause an explosion. Therefore, we should be careful of oxidizing compounds. Because at any time on heating and mixing with a reducing agent, this compound may become unstable. The oxidizer properties of potassium nitrate can be utilized as an additive to the manufacture of explosives, such as the "Black Powder" bomb used in World War I by American troops.
Reaction of KNO3
Reaction of KNO3
Potassium nitrate can be prepared by reacting ammonium nitrate with potassium hydroxide.
NH4NO3 + KOH → KNO3 + NH3 + H2OPotassium nitrate can also be prepared by reacting ammonium nitrate with potassium chloride.
NH4NO3 + KCl → NH4Cl + KNO3Or Potassium nitrate prepared by reaction of potassium chloride with sodium nitrate
NaNO3 + KCl → NaCl + KNO3
Conclusion
Conclusion
Potassium Nitrate has many benefits, many of which are widely used in the armaments sector such as bomb making. In addition potassium nitrate is also used as a fertilizer, as a nitric acid making agent, Oxidizing salts or oxidizing agents so that contact with organic materials or reducing agents should be avoided because if potassium nitrate reacts with the material it will cause a fire or even an explosion, therefore never react with potassium nitrate with an organic material or a reducing agent.
Source :
Support Scientist By Use #science tag or join @steemSTEM
Follow Me @jamhuery


Woooww.
This is goats but different to a goat other in terms of horns and color.
I loved this goat.
An animal unique and rare is rarely we get like this it is remarkable you post.
Bg neu saweu2 sigou2 bak. @mubarak
okey, upvote for every post
Ok brother you are the best in steemit..
Thank you
Join us on #steemSTEM / Follow our curation trail
Thank you for this very interesting article. It has been advertised on our chat channel (and upvoted).
The steemSTEM project is a community-supported project aiming to increase the quality and the visibility of STEM (STEM is the acronym for Science, Technology, Engineering and Mathematics) articles on Steemit.
Nice info friend!
Thanks for reading and support me @himal
meep
crazy
@jamhuery got you a $0.92 @minnowbooster upgoat, nice! (Image: pixabay.com)
Want a boost? Click here to read more!
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by jamhuery from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, and someguy123. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you like what we're doing please upvote this comment so we can continue to build the community account that's supporting all members.
nice post~ that minnowbooster goat actually resonated well with you post haha
https://steemit.com/curation/@steemwizards/steemwizards-curation-trail-day-15-and-competition-join-the-magic