Science For Growers: pH Explained as Simply as Possible
Many growers are really preoccupied with pH of their soil or water tank, but they rarely know what pH really is, except that it can be acidic, neutral or alkaline. Well, it can be due to concentration of hydrogen ions, which is the very basis of pH.
In chemistry pH is defined with this equation:
This breaks down to POTENTIAL OF HYDROGEN (one the left side) equals (in the middle) negative base 10 logarithm of the molar (or units of moles per liter solution) concentration of hydrogen ions (on the right side).
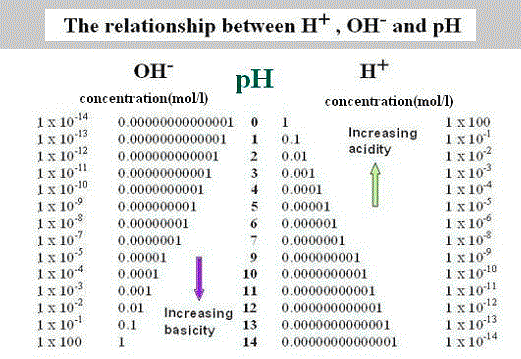
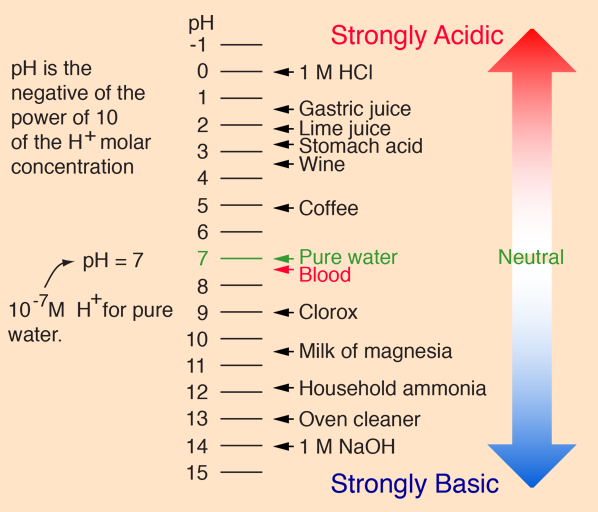
This equation was coined in 1909 by by Danish biochemist Søren Peter Lauritz Sørensen, and it measures the acidity or basicity of the solution on the logarithmic scale (nonlinear scale used when there is a large range of quantities), that spans from 0 to 14, where each single unit corresponds to a ten-fold change in hydrogen ion concentration.
To be clear, solutions over 7.0 are considered alkaline, and solution below are considered acidic with those that are exactly 7.0 considered neutral.
The interesting is, that pH scale is theoretically open-ended, but it's been SIMPLIFIED for the clarity as an extremely acidic solution can have 100,000,000,000,000 times more hydrogen ions than a very alkaline one, and vice versa... a strongly alkaline solution can have 100,000,000,000,000 times more hydroxide ions than an acidic one.... which comes down to very advanced maths, that might cause a severe headache if you're not trained for it!
On the top of that the concentration of both ions in soil or water is shifting all the time, so there's no way to get a fixed number for more than a few seconds!
According to the above-mentioned, pH is all about the concentration of hydrogen ions (H+). But where do they exactly come from? Well, they come mostly from water (H2O), which splits up in natural conditions into hydroxide (OH-) and hydrogen (H+), which quickly forms so called hydronium (H3O+), commonly referred to as hydrogen though.
This basically means that in order for the solution to have pH of 7.0 there has to be an equal number of of hydrogen and hydroxide ions. If the number of hydrogen ions go up, then the solution becomes acidic. And when the number of hydrogen ions go down, then the solution becomes alkaline.
But soil has much more going on that just catching hydrogen ions from water (mostly rainwater). In fact soil pH in nature is mostly regulated by decomposition and mineralisation of organic matter as well as its geological structure (strata of calcium, magnesium, aluminium, sodium or iron), and root respiration of plants growing in it.
This is easier said than done. Adjusting pH in hydro solution is for example quite easy, which is why hydro growers rely on pH ups and downs all the time to control the absorption of nutrients. However, in soil it's much trickier as soil pH is regulated by another factor called BUFFERING CAPACITY.

What it basically means is, how much of acid or alkaline solution a given soil can soak up before its pH starts to shift. If buffering capacity is high, then changing it becomes a real challenge, and if it's low, then it's much easier.
As a rule of thumb, high clay and humus (peat belongs here too) content will up buffering capacity of soil significantly making a forced pH swing quite difficult!
In organic soils it's almost impossible to change it permanently as soil microorganisms will always fight to keep it in the optimal range to thrive, but even in soilless mixes a desired value may be quite hard to set for longer than a couple of hours due to hydrogen exchange.
In fact, during cannabis' life regulating it with acidic or alkaline solutions is pretty much pointless - unless you want to kill your plant to prove a point - as it'll always bounce back, and the only way to set it in desired parameters is to correct the soil before planting and test it for base saturation (the test has to measure hydrogen saturation).
Moreover, measuring runoff to figure out pH is a VERY WRONG way to do it as percolating water won't reflect THE ACTUAL pH OF SOIL, but only its own pH after passing through the medium, and the only way to get a more or less accurate reading is THE SLURRY TEST!
Photo credits: 1st from the top is Gorilla Glue grown organically indoor by Ilikemsticky, 2nd from the top is Tangie grown in #highbrix soil by Doc Bud. Both pics courtesy of 420Magazine. Tables credited directly
Did you like it?
Follow, upvote, resteem
💚 💚 💚 💚 💚
What is an air stone? And what does it mean to bubble your solution? Ive read some things about making a tea solution and kinda over my head since I’m very new to this.
That's an air stone. It's used for bubbling stuff!
I just read that using an air stone to bubble a organic nutrient solution will raise the ph to the desired level. Specifically the nutrient solution I’ve been using!! No wonder why shit always fucks up for me in flower, because i rarely use fertilizer in veg.
I can’t wait till i do a side by comparison of your recipe to mine! Should be an interesting experiment.
When you get all the ingredients, hit me back! You'll need to cook the soil after you mix it, which means leaving it for 6 weeks in hot & humid conditions!
What if it doesn’t get hot nor humid where i live?
You'll need to add water, no biggie :)
Really wish i could do an outdoor grow. I live in the damn salad bowl of the world but am forced to go indoors! Lol
County regulations?
I love when scienctific evidence confirms my intuitive understanding. I have adjusted ph zero times, & measured ph zero times. A soil test would be smart to do at some point, but even with a soil test you get a small picture of what’s really going on in the whole bed.
Great write up
Never measured it either... really pointless in organic soil. Soil test is the way to go if you want to dial things in though, especially if you're getting weird deficiencies that you can't put your fingers on!
I’m starting to discover ph-ing can really do more damage then good. It seems ph-ing is really for chemicals and hydro. I recently bought a ph kit but just discovered i need to mix my solution I’m using 24 hours prior and let it aereate
Yes it is. It's valuable as a parameter in soil, but once it's been tested and the numbers are ok, you'll be ok all the way... providing you don't use too much chemical shit as most salts will acidify your soil with time.
Awesome information yes PH is critical for fat buds.
It's slightly more complicated than that :D But base saturation is very important indeed :)