Fundamentals of Adsorption and Activated Carbon
Hey steemians, how was the weekend. I hope you had enough rest to replenish your strength cos it’s another week again.
Let me not waste much time and go into the topic of today.
First, let me ask you a question. Perhaps you do notice a small pack containing some round balls in your new shoes or bags. Do know what that is? Well, that’s silica gel. It has affinity for moisture and it is put in shoes and bags to rid them of moisture. But what is the principle behind silica gel’s affinity for moisture? That we will look at in this article. The phenomenon is called Adsorption.
DEFINITION
Adsorption is a phenomenon whereby molecules, atoms or ions from a solution, gaseous, or liquid medium adheres to the surface of another material. The material or substance which is adsorb is called the adsorbate, while the material which provides the site for adsorption is called the adsorbent.
There are various types of adsorbents which can either be liquid or solid. Some common adsorbent are – Silica gel, Clay, Zeolite, Activated alumina, and Activated carbons. We will focus on activated carbons, how they are prepared, their types, some important properties, and applications.
Many find the concept of adsorption confusing with absorption. However, you should know that both are two different things with a lot of distinctions. Let me quickly give you the answer. I am sure this will give you a clearer picture of what Adsorption really is.
DIFFERENCE BETWEEN ADSORPTION AND ABSORPTION
Firstly, take this simple analogy. Imagine you are to meet your date on a very good afternoon. You are putting on your sleek suit and that new shoe you just purchased. You are now set for the date but unfortunately, things went south – As you were waiting for your cab, it began to rain. Gosh!!!... I can’t go wet for a date, you thought to yourself.
Fortunately, there’s a building nearby with a front cover. Of course, you have to take cover under it. Thank God for you, at least you now have a place to prevent yourself from becoming dripping wet, but you have to stay glued to the wall to avoid the wet wind from the rain. Suddenly, the owner of the house comes out –but why?
Oh no, you were resting on the door unknowingly and had hit it in the process. But to your surprise you, were invited in by the owner who came to inquire- How kind. Well, your only prayer now is that your date does not leave. So for your sake, I hope so. We should go back on course.
Let’s see how your ordeal relates to with the concepts in question. Taking cover at the front of the house and staying glued to the wall is like adsorption. You had no access into the house at that point in time. Your best choices was to adhere to the wall to prevent your dress from the wet wind. However, you were fortunate to be invited in. Now, entering into the house can be likened to absorption. That is, you were absorbed by the house and you became an integral of the house.
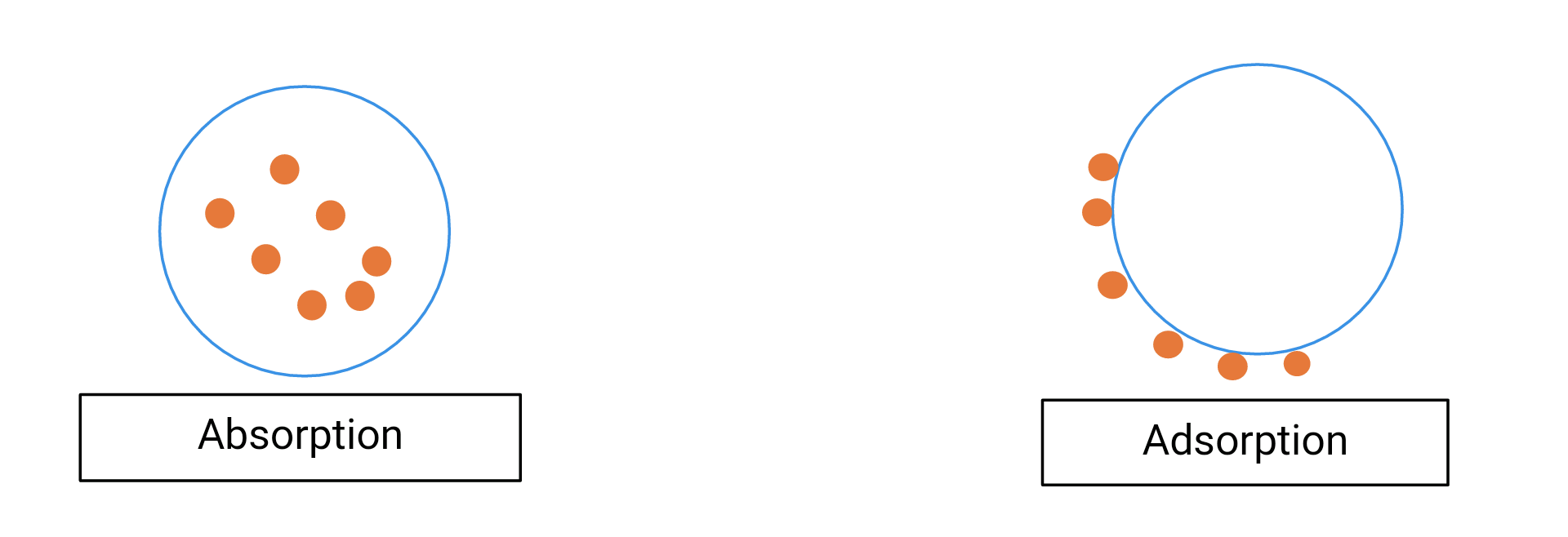 Image created by me (@temitayo-pelumi)
Image created by me (@temitayo-pelumi)
Thus, Absorption deals with the bulk of a material. The molecules, ions or atoms absorbed travels beyond the surface of the absorbent into all spaces present within. That is, Absorption is a volume phenomenon. Adsorption on the other hand is a surface phenomenon as the species adsorb only stays glued to the surface of the adsorbent and does not go into the bulk of the adsorbent. Now I hope you dig.
TYPES OF ADSORPTION
Physical Adsorption
Physical adsorption is also known as physisorption. In this case, Van der Waals forces holds together the molecules, ions or atoms of the adsorbate to the surface of the adsorbent. In physisorption, multiple layers of the adsorbate are formed on the surface of the adsorbent. It occurs at low temperature, usually below the boiling temperature of the absorbate and the amount of adsorbate adsorbed decreases as we increase temperature.Chemical Adsorption
Chemical Adsorption is also called chemisorption. In chemisorption, there exist a chemical bond between the molecules, ions or atoms of the adsorbate and the surface of the adsorbent. Thus due to this nature of chemical adsorption, the forces of attraction between the adsorbate and adsorbent are stronger than in physical adsorption. In chemisorption, a single layer of the adsorbate forms on the surface of the adsorbent.
Chemisorption may occur at any temperature. The amount of adsorbate adsorbed increases at first with increasing temperature and then begin to decrease after a critical temperature is reached.
ACTIVATED CARBON
Activated carbon, also called activated charcoal sometimes is an adsorbent which is designed to have a large number of microscopic pores which serves as site for adsorption. Activated carbon differs from ordinary charcoal in that it has more pores and can be prepared to meet a particular need. What makes them different is the process of activation, which give activated carbons more pores and thus large surface area for adsorption.
PREPARATION OF ACTIVATED CARBON
There are two methods through which activated carbons are prepared. One is Physical activation and the other is chemical activation. Activated carbons are prepared from carbonaceous (carbon-rich) materials, such as wood, coconut shell, coal and so on. Let’s look at these methods of preparation.
- Physical Activation
In Physical activation, also called gas activation, the raw material for the activated carbon is first made to undergo pyrolysis. Pyrolysis is a thermal process that is used to transform a carbonaceous matter into a substance with high carbon content by driving off unwanted substances such as moisture or volatile matter. During this process the raw material is heated to temperatures in the range of 600 – 900 oC, in the absence of oxygen to form a char. Prior to pyrolysis, the material is first prepared into the appropriate size.
After pyrolysis, the next step is the activation operation itself. During activation, the charred material is exposed to hot oxidizing environment such as oxygen, steam or carbon dioxide at a temperature ranging from 800 to 1200 oC.
- Chemical Activation
In chemical Activation, the raw material is first treated (impregnated) with a suitable acid, base or salt. Substance that are used includes phosphoric acid, Sodium hydroxide and Zinc chloride. The mixture is then heated to a relatively lower temperature, usually in the range of 450 - 900 oC. Chemical activation is faster due to the lower temperatures than physical activation.
Note: The preparation process of activated carbon depends on the application. Consequently the preparation is always designed such that the resulting activated carbon, meets the requirements of the application.
SOME PROPERTIES OF ACTIVATED CARBON
- Surface Area
Perhaps this is the most important property of activated carbons as it is crucial to adsorption. Activated carbons have very large surface area. Usually it ranges from about 500 to 1500 m2/g. That is if you are given one gram of activated carbon, the surface area could be as large as 1500 m2. The least possible size of a football pitch is about 4050 m2 which obviously 3 grams of a 1500 m2/g activated surpasses – isn’t that fascinating? Moreover, there are cases in which the surface area is much more than this stated value.
The more the surface area of an activated carbon the higher the effectiveness of adsorption and vice versa.
Total Pore Volume
We have noted that activated carbons have tiny pores within them. The total space which is occupied by these pores for a unit weight of the activated carbon is called the Total Pore volume. The Total Pore Volume is given in milliliter per gram of activated carbon (ml/g). The total pore volume is a function of the raw material as well as the preparation method. As with the surface area, the larger the Pore volume the more effective the adsorption. However, it is essential that the pore size matches the size of the molecules, atoms or ions of the species to be adsorbed.Pore Size Distribution and Pore Diameter
The pore size distribution is a graph showing the spread or distribution of the pores sizes present within a given gram of activated carbon. This is simply done by taking all the sizes of pores existing in the activated carbon and then plotting them against their frequency of occurrence (Statistical stuffs). Perhaps I should give an example.
Imagine in a given class, 5 students had A – grade, 15 had B, 7 had C, 3 had D and none had E nor F – grade. Now, if I ask you to plot the graph of these grades versus the number of people that had them. I guess now you understand the concept. Now let’s talk about the Pore Diameter.
Based on the diameter, Pores could be Micropores, Mesopores or Macropores. Micropores are designated to have diameters lesser than 2 nm. The diameter for Mesopores is in the range of 2 nm to 50 nm, while Macropores have diameters larger than 50 nm.
Iodine Number
The Iodine number of an activated carbon is an indicator of the micropore content and thus determines the ability of an activated carbon to adsorb smaller molecules. The higher the iodine number the more the micropores content in such activated carbon. It is measured in milligram of iodine adsorb by one grams of the activated carbon (mg/g).Molasses
The molasses is the measure of the mesopore content of an activated carbon. It is the ability to adsorb relatively larger molecules.
TYPES OF ACTIVATED CARBON
- Powdered Activated Carbon
Powered Activated carbon (PAC), as the name implies is activated carbon that has been crushed and milled into fine powder. Their particle size ranges from about 0.5 – 15 nm. They are advantageous in terms of their flexibility as well as low manufacturing cost. In terms of flexibility, I mean the quantity used for an application can be easily altered to meet changing conditions of such application.
Due to their fine nature, a larger surface area to volume ratio is presented and this makes them suitable for applications where diffusion is less. As such, they are suitable for Liquid-phase adsorption. PAC are not always regenerated for reuse due to the difficulty associated with their recycling.
Note: surface area to volume ratio, measures the ease of accessibility of the adsorbent by the adsorbate. For instance, if you are given a sliced-loaf of bread and an unsliced one. Let’s say I want you to apply butter to the whole bread without making extra cuts. Which will be more accessible? or which will get more butter?
You dig?
Granular Activated Carbon
Granular Activated Carbon (GAC), are activated carbons with sizes relatively larger than that of PAC. The have haze granule sizes which ranges from about 2 – 5 cm. Due to their larger size, they have relatively low surface area to volume ratio. Consequently, they are best suited for systems where diffusion rate is high such as in gases and vapours. However, they can be used for liquid-phase adsorption as well. GAC are readily regenerated for reuse.Extruded Activated Carbon
Extruded Activated Carbon (EAC) are those prepared by extrusion operation. I explained the concept of extrusion in my post on additive manufacturing here . In EAC, fine powered activated carbon is mixed with a binder and then extruded. The result products of the extrusion operation are cylindrical shaped activated carbon which is hard by nature and can be used for heavy duty operations.
Extruded activated carbon find application in the Evaporative emission control system of automobiles.
USES OF ACTIVATED CARBON
The uses of activated carbon is diverse but all works based on adsorption. We can conclude that activated carbon is are utilized for separation. That is, they facilitate the removal of one substance from the other. “What is removed from what” now depends on the area of application. This in turn will determine the kind of activated carbon to be used and thus the preparation of such activated carbon. Remember, I noted that the activated carbon can be prepared to serve a particular need.
One major application of activated carbon is in water treatment. In terms of the source of the water and its purpose, there are various water treatments most of which employ activated carbon at one point or the other. Activated carbon is used in drinking water purification. It is used to remove substance affecting the taste and colour of water to serve the purpose of drinking.
Treatment of Service waters such as waste water from chemical plants, boiler feed water in a steam power plant etc. also employ activated carbon. It is necessary to treat waste water by the removal of hazardous substance in order to ensure that the water is eco-friendly. Boiler feed water are need to be of high quality to prevent rust of plant equipment such as boiler and turbines. This is achieved by an appropriate water treatment process in which passing through activated carbon is one step.
Activated carbon also find application in the evaporative emission control systems in Automobiles. This is a system put in place in modern cars to trap and hold petrol Vapour from the fuel tank which would later be burnt in the engine. This prevents the waste of fuel (i.e. it improves fuel economy) and environmental pollution that will result if vented into the atmosphere.
Activated Carbon also finds many medical uses such as in dental application where it is used to whiten the teeth. It is also used to treat alcohol poisoning and prevent hangover.
CONCLUSION
We have seen what adsorption is and we were able draw a dividing line between the concept of adsorption and absorption. I’m sure now you know what adsorbents are and some types of it, particularly Activated carbon. We have seen how they are prepared, some important properties as well as their applications.
Thanks for reading through.
- HAYCARB: Activated Carbon Basics
- Separation Processes: Adsorbents
- CHROMATOGRAPHY TODAY:Adsorption, Absorption and Desorption — What’s the Difference?
- Water Technology: PROFESSOR POU/POE: ACTIVATED CARBON
- Chemistry Learning: Adsorption
- Wikipedia: Activated Carbon
- BBC Sports: Pitch Dimension
- Dr. Axe:Top 10 Activated Charcoal Uses & Benefits





@ temitayo-pelumi visit:
https://steemit.com/contest/@nitego/400-followers-contest-winners
To claim your price
I am glad to see another good article of yours. My first ever article on Steemit was an article about activated carbon in car filters to limit the level of PM2.5 in the busy road conditions, and I also use a huge activated carbon filter to filter the impurities and smell out of my drinking water.
The applications are also extended to space or Mars life support systems. Filtering or refiltering used water and gases from a Mars colony would tremendously help the development.
Keep it up and you will organically grow from here! And keep your eyes on the tools for analytical purposes :P
Thanks for your support @alexdory.
Do you know i was reading adsorption as absorption until i got to the point where you differentiated them...,
Your analogy made it quite simple to understand howbeit lovely.
Can you say cassave flower(garri) is a form of adsorbent? It is usually used to reduce the state of been drunk
I would not think that bro. Garri absorbs, it doesn't adsorb.
Hmmm, Thanks for clarifying that.
You are welcome.
In chemical adsorption, gases are held to a solid surface by chemical forces that are specific for each surface and each gas and they also occur usually at higher temperatures than those at which physical adsorption occurs.
Thumbs up bro.
Yeah. You are absolutely correct. There's a limit to the temperature in which physical adsorption can occur.
Thanks for your contribution.
You're welcome... Always
How can I use it to whiten my teeth?
By the way, did I tell you this is a very nice post? Well it is.
To whiten your teeth. You need to get a powered activated carbon and use it to brush your teeth. It's a simple as that. It will adsorb substances responsible for the brown colouration.
Thanks for your comment.
Jesu... I hope the black color wouldn't affect the teeth?
No... It will wash off.
I will try it.
Yeah... You should.
Wow, well-done, I've only sparingly heard of Activated carbon, but the way you dissected the whole concept just makes it easier to pay attention. Bravo
I glad it was explicit to you. Thanks for the comment.
Quite insightful. Well written. Can we use activated carbon for bleaching?.... Fabrics I meant...
I'm not ruling out the possibility but I will say is not that simple. Diffusion is key in adsorption and that is not readily available with just a fabric and Activated carbon.
OK. Thanks for the response.
Beautiful analogy to explain the difference between adsorption and absorption. Very good effort.
Feel free to engage other authour in their comment sections and you can as well join the steemstem discord channel to interact with them.
Thanks for dropping by @gentleshaid. I already joined the steeemSTEM discord server.
Thanks for your kind comment.
Being A SteemStem Member