Nucleation process in crystallization technology;polymorph preparation.#5
Hi everyone, good to be here again.I hope that you are doing fine.In case you missed the last post, you can check it here
For some time now, I have being discussing crystallization technology as it is applicable to pharmaceutical industry.You might feel like, or even ask yourself how does that concern you. But i think that as a scientist or if you are not one, should have a basic idea of how those guys in the pharmaceutical industry laboratory produce the drug that you consume.
You know that this is what your doctor prescribed for you, I even guess that some are on drug prescription right now as you are reading.
This post is just meant to enlighten us on some procedure been followed in the pharmaceutical industry. Sit down and enjoy the post while it lasted.
Crystallization from a supercritical fluid.
A substance is termed as supercritical fluid when it temperature and pressure is beyond it's critical point.At that instance, very clear liquid and gaseous phase is in oblivion. This fluid can effuse or pour out through solids like gases and solvate materials like a liquid.

phase diagram showing critical point of supercritical fluid
By Matthieumarechal, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=4623701
Also, alongside the critical point, a little variation in pressure or temperature leads to a large change in density, giving room for many properties of a supercritical fluid to be adjusted.This fluids are appropriate as a replacement for organic solvent in various process ranging from small scale laboratory to large scale industrial process.
Supercritical fluid are used as a medium for the production of new crystalline forms of pharmaceutical co-crystals.The technology provides a new stage that gives room for single-step formation of particle that other known traditional methods are incapable of generating.
The isolation of pure and dried new co-crystals is obtainable due to the distinctive properties of supercritical fluid by leveraging on the various properties of the fluid.
Supercritical fluid technology present a means for the preparation of crystalline particles which consist a substance in solvated form. This is achieved by the dissolution of the substance in an initial solvent, and then introduce the solution with a supercritical fluid into an apparatus, in which the fluid contain an anti-solvent and a secondary solvent which is water.
Mostly, the anti-solvent use for this technique is carbon dioxide a good example of supercritical fluid which is completely saturated with the secondary solvent.
This invention also yield formulations consisting of particles made according to the process consisting one or more pharmacologically active substances or active pharmaceutical ingredient (API) and one or more of other pharmaceutically acceptable additives that is use for such drug formulation.
Crystallization using capillary tube
A good example of a drug whose polymorph is obtained through this technique is nabumetone.Crystallization studies carried out using capillary tubes produced a metastable polymorph of nabumetone. The X-RAY elucidation of a selected high-energy form of a single-crystal of the drug was compared with that of a low-energy form of the same drug.Grinding of the metastable form with a low-energy form in a solid state converts the high energy form to a low energy form.
The existence of fragile C-H•••O interaction contribute to the unsteadiness of the metastable form, when it is placed side by side with the thermodynamically more stable state.
Apart from nabumetone, some other drugs can be crystallized into their metastable polymorph when solution of such are evaporated from capillary tube.
X-RAY powder diffraction and thermal microscopy is used to differentiate between metastable polymorph and more stable form of such drugs.
X-RAY examination of both polymorphs shows the existence of comparatively weak non bonding interaction in the metastable state which is known to be responsible for the flunctuation of the polymorph.
Nabumetone which is a nonsteroidal drug used to treat inflammation under trade name such as Relafen, Gambaran and so on is known to have two polymorphs.The crystal form of one of the polymorph has been known to show lower stability yet dissimilar only in frail intermolecular interaction from the the other polymorph.
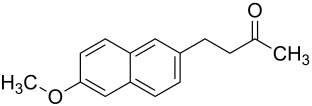
Nabumetone.
By Emeldir (talk) - Own work, Public Domain,
The crystal arrangement of the second polymorph of nabumetone differ from the known polymorph In their weak interaction.The crystal arrangement of the new polymorph is quickly transformed to the known polymorph form under favourable kinetic condition.
Crystallization under the influence of tailor made additives
The presence of additives similar in structure with drug whose polymorph is to be made influence the formation of such polymorph.
Tailor made additives when incorporated into insoluble polymer, reduces the time needed for crystal formation.This method give room for the synthesis of crystal of compound that may be resistant to form crystal.
The primary phase of crystallization, nucleation, can be fast rack when a surface is applied to quicken the aggregation of molecules by heteronucleation.
This method is among various method employed for heteronucleation, and it has proven beyond doubt that it's a reliable method for discovering polymorph, using several insoluble polymers as director for crystallization in obtaining unique solid forms.
The principle behind this method is that interaction between functional group at the crystal and polymer interface account for directing and steering the nucleation of various crystal phases while using a particular polymer additive.
Exposure to organic solvent.
Formation of polymorphs depends also largely on solvent used.
The type of polymorph formed depends on the values of hydrogen bond donor ability (HBD). For example, the formation of form l and form II of prasugrel hydrochloride, depends on wether the solvent used has high or low HBD. Form I will be formed if the value of HBD of solvent use is low and form II will be formed if the value of HBD is high.
The kinetics and thermodynamics explanation for the solvent impact could be explained by the use of data obtained in the solubility and nucleation experiments.The solubilities of form I and form II gotten through gravimetric procedure, and an equation which is gotten from the linear free energy method for predicting solubility was used to harmonize the solubility of form II.
The value of solvent hydrogen bond donor ability also influence the solubility of drugs.Polymorphs obtained after dissolution are also closely related to the solvent in use.
The interaction between solute and solvent also influence the nucleation rate of polymorph formed.
In the case of prasugrel hydrochloride, it was observed that form I crystallizes if the van der Waals force influence the interaction between the solute and the solvent molecules, while form II polymorph is likely to nucleate and grow into crystals if it's the HBD that influence the intermolecular interaction between solvent and solute molecules.
Vapour diffusion technique
This technique is applied when small quantity of sample is under use.A drop of the concentrated drug which is already in solution is placed under the cover slip of a microscope slide.The cover slip which contains, the drop is then covered with a silicon oil in a solution that is highly concentrated with precipitant.
Because of presence of high precipitant concentration, the latter has lower vapour compared to the drug solution. This leads to diffusion of the solvent from the drop towards the reservoir and successive crystallization of the Active Pharmaceutical Ingredient (API) which may take hours or even weeks.
Thanks for stopping by.
You can leave comment behind and let's debate it.
Keep on steeming.
Note that all images are from free source.
As a follower of @followforupvotes this post has been randomly selected and upvoted! Enjoy your upvote and have a great day!
Good post, thank you for enlightening and educating me more tonight. hoping to read more of your post.
Thanks for stopping by, @ugonma, expecting to see more of you. 👍
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Steepup from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
Being A SteemStem Member