Our sky's interaction with solar radiations --- The ozone layer, it's depletion, and what we could do to save our planet.
We know that energy from the sun (Solar radiation) heats up the earth's surface and its atmosphere. and that earth's surface in turn, absorbs some of this heat energy, liquids evaporate and things kept outside gets hot. Hence, the earth becomes heated up, and radiates (give off) heat too.
One question on my mind was how the atmosphere comes in on all of this. Is there a slight chance that our sky could absorb/retain, or even reflect heat energy?
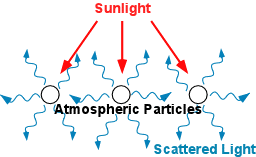
Actually, it does, and even though it's mostly made-up of air and moisture, the atmosphere interacts with solar energy much more than the earth's surface does. Some cool way science could prove this is the Rayleigh absorption and scattering of light (which is the reason why the sky is blue), as well as other natural phenomenon or effects created by our sky's interactions with several other gases, such as CO2 and O3, which is the scope of this article. Also, clouds up high in the atmosphere reflects and absorbs some of the sunlight by blocking them from reaching the surface. Little wonder why cloudy days feel a bit cooler than sunny ones.
Today, I'd like to talk about our atmosphere's interaction with solar energy, specifically focusing on UV energy and the ozone layer, and how much human activities have affected the zone and the climate in general. Do stick around.

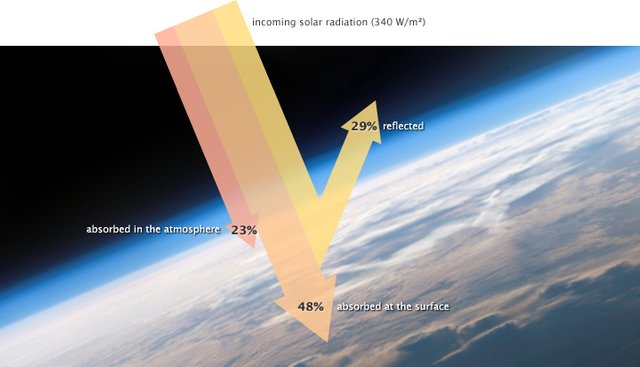
So, having established the fact that our atmosphere interacts with solar energy pretty much more than the earth.s surface, and it isn't just a pretty name, it's important to know just how much interaction we're looking at, given the continual influx of radiations from space. Here are three things we need to know about our atmosphere and radiations:
- Absorption:
According to EESC, Columbia, just about 17.5% of the radiation entering the atmosphere is absorbed(converted to heat energy in a body) at the various levels in the sky. The rate at which this happens depends on the altitude in question, as well as the type of gas molecules present in the air. Since oxygen and nitrogen make up the bulk of the atmosphere, and are diatomic with 2 electrons in their outermost shells, they absorb more of this energy (with oxygen absorbing a wider range of the electromagnetic wavelenghts), and this weakens the bonds between their two atoms, and they break up to single atoms flying around the atmosphere at full speed. So we have it as:
O2 + Ultraviolet = O + O.
O3 + Ultraviolet = O2 + O
This occurs mostly at the uppermost layer of the atmosphere, and would account for that part of the atmosphere being very hot, -being that we already know that particles get hotter when they absorb radiation-. Absorption is mainly due to the presence of the ozone.
- Reflectivity:
Our atmosphere reflects about 35% of incoming solar radiation back into space, with clouds causing a massive 24% of that number and the other 7% caused by scattering. leaving about 4% of that to fall to the surface. The process of scattering occur when very small particles and gas molecules diffuse parts of these radiations in random directions without altering the radiation's wave. consequetly, more than half of the scattered radiation is returned into space. I'd say good riddance! Also, with all the scattering, the cloud reflects a good portion down to the earth's surface too.
Transparency: - letting it pass through-
The atmosphere allows about 22.4% of the radiation it receives through it to the surface where it is either reflected back (4%), or absorbed by land, water, rocks, and so on.Light Emission:
The atmosphere also emits light after absorption. The famously called Aurora or Northern lights, is an interaction between solar particles and the constituent gases of air in the ionosphere. these supercharged solar particles excite the electrons of gases (mostly oxygen and nitrogen), and we see beautiful curtains of colours in the night sky. They occur closer to the polar regions because the earth deflects these incoming solar particles to the poles, where they enter the atmosphere.

The Ozone layer --- Our protector from the extremes.
Introduction.
As i mentioned, it isn't uneventful for earth to get cosmic radiations from time to time, since we are directly in the path of the sun in the solar system. Some of these radiations are powerful enough to cause tons of damage to Earth's ecosystem if exposed to them over a long period of time. One really familiar name is the Ultraviolet light (UV), which has higher energy than visible light. Hence, we cannot see it. Excess UV on the skin could lead to cancer, sunburn, damaged eyesight, destruction of plastic materials and plants, etcetera... As such, our Earth has a defense system set up somewhere in the sky for keeping just a bit of UV from reaching earth's crust. We call it: The ozone layer.
What's Ozone and why does it have a layer all to itself?
The ozone is basically an oxygen atom rearranged in a different way. It has a characteristic strong spark-like smell (the same smell you get from electric sparks), and it is blue in colour. How it is formed is that an extra electron of oxygen joins itself to a regular oxygen gas molecule, and poof! what we get is the ozone.
The fancy scientific word for the reorganization of an element's electrons to give a new material is called Allotropy, and the ozone is an allotrope of oxygen with three electrons instead of two. With the symbol O3.
This ozone is found in trace quantities in different layers of the atmosphere, with some layers having more ozone than the others. "Trace" because The amount of ozone in the atmosphere is in bits and pieces as regards quantity, with only about three to four ozone molecules present in every 10 million air molecule.
The region in the atmosphere where more ozone molecules are present is what we call the ozone layer, and it was discovered by French physicists Charles Fabry and Henri Buisson, in 1913.
As already explained, the concentration of the ozone varies in altitude from layer to layer, with most of the ozone on earth (though still little in quantity,) accumulating in the lower part of the stratosphere (the layer of the atmosphere at a distance of about 15 to 30 km from the earth's surface). Even though the ozone molecules are constantly breaking apart, the whole reactive process is in a complete cycle. Hence, the reactions balance and fix itself to repeat the process all over again.

How the ozone layer works.
If you've never gotten sunburns on a really hot and sunny day, then you have the ozone layer to thank for that. It doesn't get called the ozone layer for nothing. Despite it's relatively small quantity in the sky, the ozone layer single-handedly carries out the all important function of shielding the earth from harmful cosmic radiations and excessive UV light from the sun.
Think about how hot and desolate the planet would have been if all the UV entering the atmosphere reaches earth's surface. It would have been disastrous, as a lot of damages would be done within a short time. For one, UV could result in the inhibition of the reproduction of phytoplankton, which of course, are at the bottom of earth's food chain. also, there's excessive heat, blindness, skin cancer and sunburn amongst others.
The ozone achieves this through the principle of absorption and molecular disintegration. Here's how:
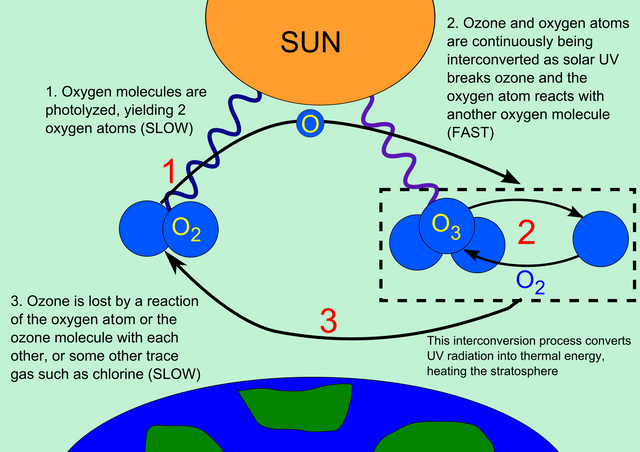
The UV in the atmosphere gets used up to disintegrate the ozone molecule, which then comes together again to form the ozone
We know the ozone to be a bogus version of the diatomic oxygen molecule with its less-stable 'triatomic' structure. Since it is slightly unstable, this allows them to break apart when struck by ultraviolet energy. The molecules of ozone absorb UV and converts it to heat energy, which severs the already weak molecular bond existing between them. Thus, producing three individual oxygen atoms. These freed oxygen atoms repeat the cycle by bonding with each other again to create more oxygen and the ozone. So it is okay to say that the formation of the ozone is a very clever 2-step process, -since it consumes UV energy in breaking apart, converts it to heat energy and then collides with itself to recreate more ozone, ready to to consume even more UV!.-
It is worth mentioning that this process of molecular disintegration of the ozone, increases the temperature of the atmosphere slightly as the heat energy formed is given off.

Human activities and the ozone layer. --- Depletion
At the troposphere where we exist, Ozone could pose some really serious pollution as compared to high up there in the stratosphere where it comes in handy, protecting all of life's forms from excessive UV that's constantly bombarding the atmosphere from the Sun.
However, human activities does play a big part in the depletion of the ozone, as some industrial processes and consumer products leads to the emission of ozone-depleting substances (ODS) into the atmosphere, with the halogens being the major culprits!. In the 1970's, Scientists found that certain man-made chemicals were disrupting the ozone cycle. A lousy atom of chlorine from a massively produced compound at the time (Chlorofluorocarbon), had the habit of knocking off the extra oxygen atom in the ozone, leaving behind the regular and powerless oxygen gas O2 against UV. And what's more, the same rogue chlorine atom could repeat this over and over again, and could damage as much as 100,000 ozone molecules. At such a rate, no new ozone molecule is formed just in time to counter that effect. Basically, it disrupts the process, and from what we know so far about our guardian - the ozone, that's not a good thing to hear.
Well, we did discover the problem, but since the mass discontinuation of the production of chlorofluorocarbon and Bromofluorocarbon by world leaders in the Montreal protocol, 1987, There has been other sources of ozone depletion too, as we've seen a steady decline of about 4% every decade for the past few years, with a whooping 85% of that change effected by human activities.
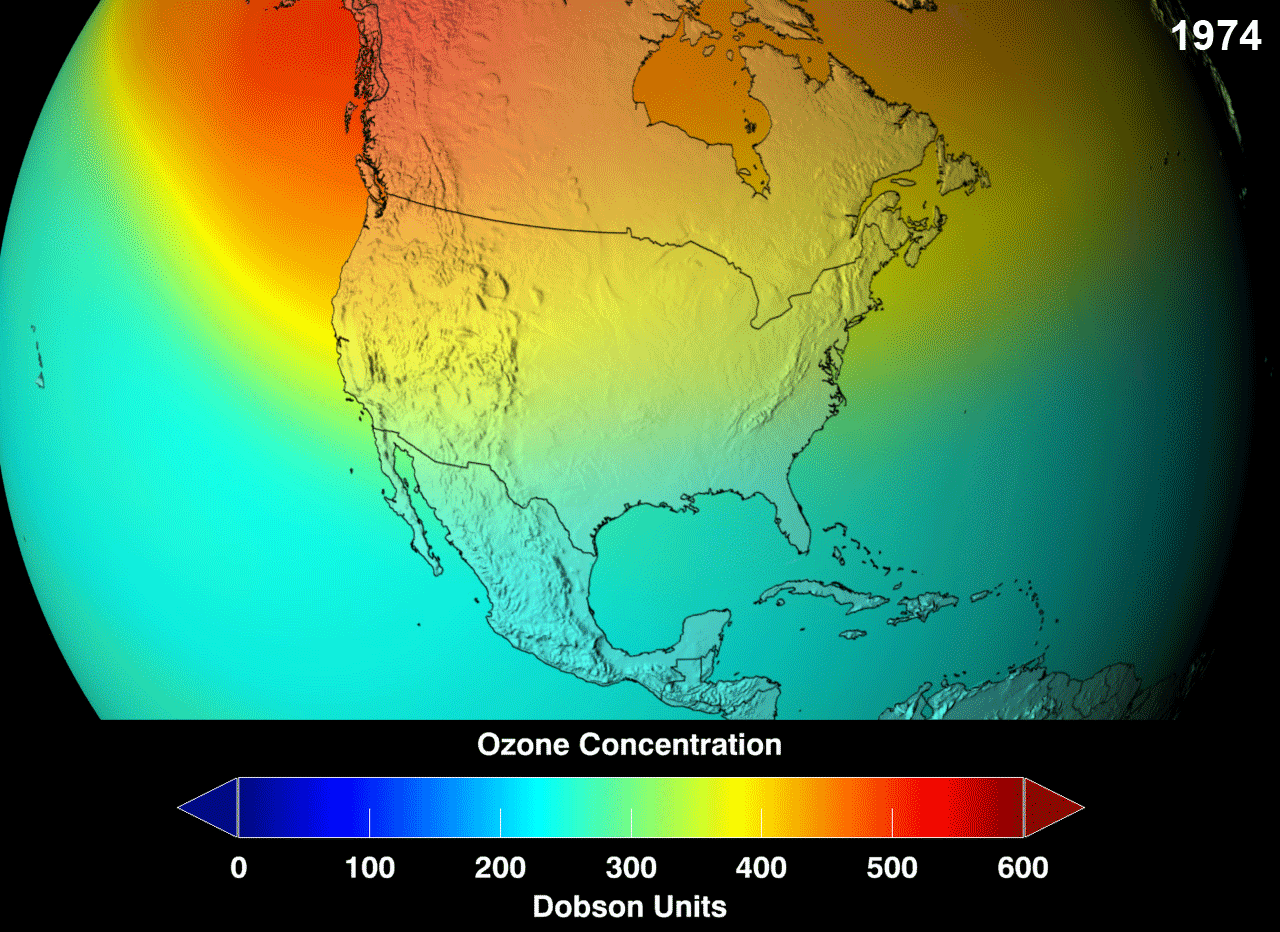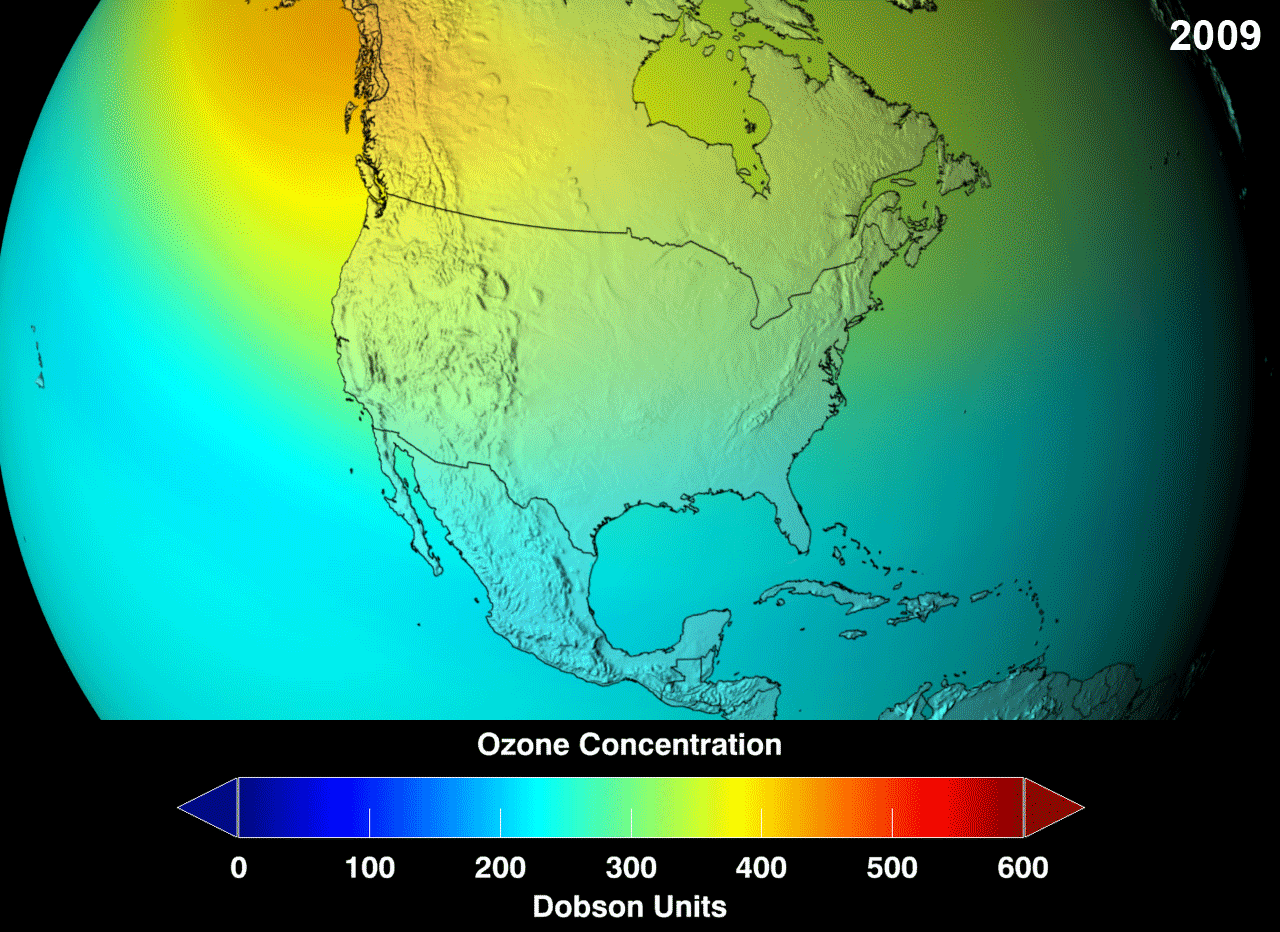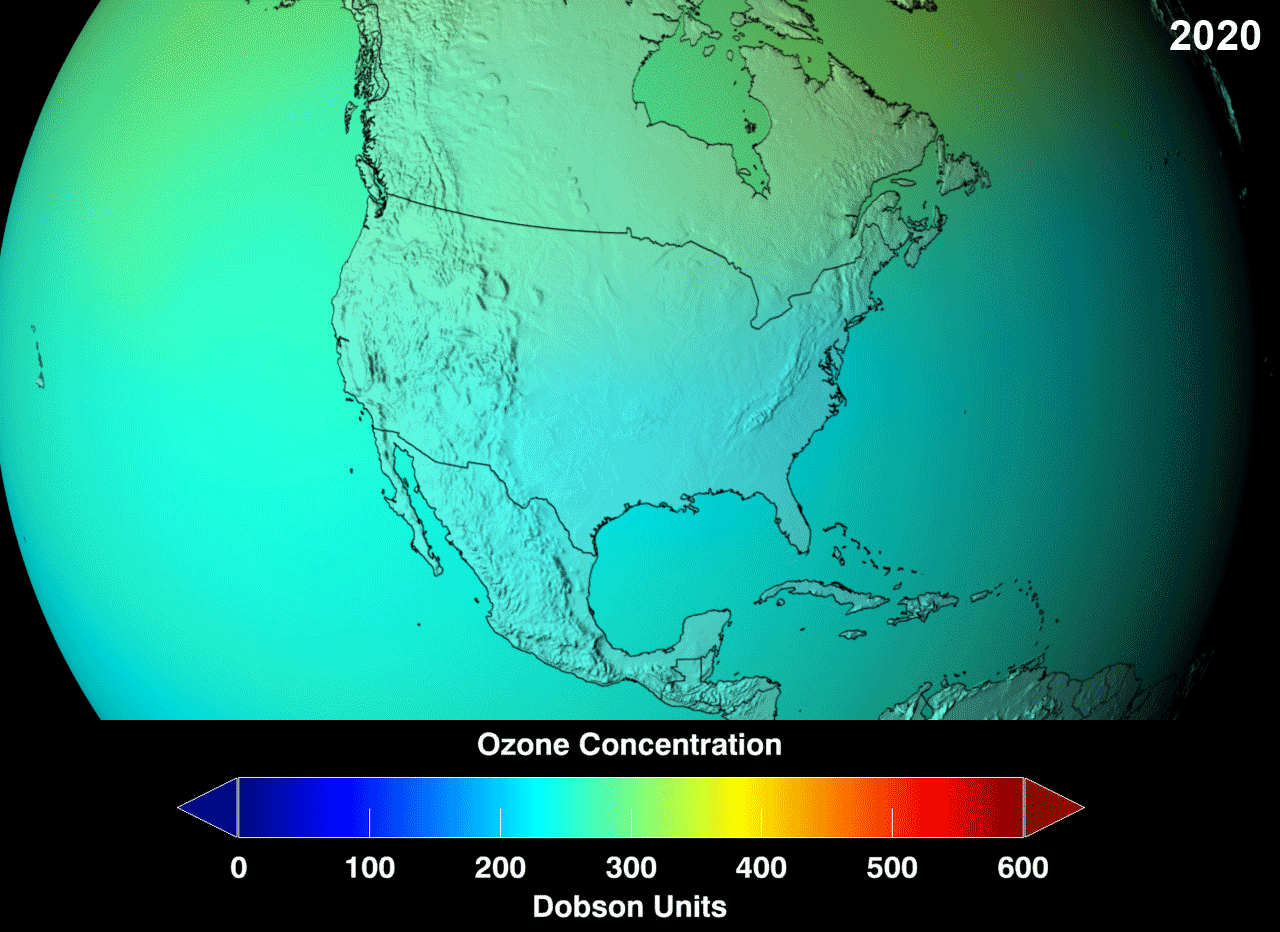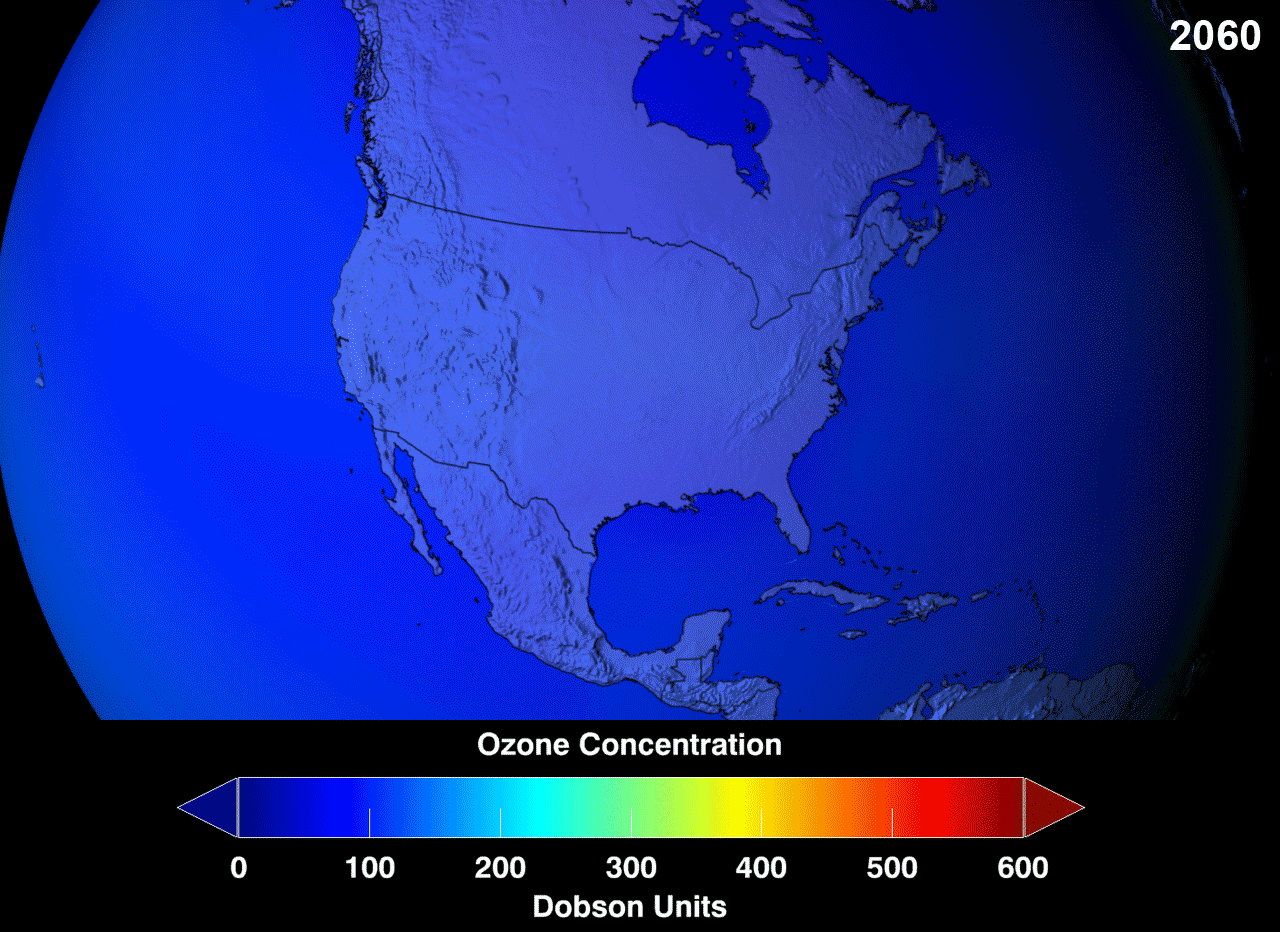
Presently, there is a "hole" (err, not an actual hole, but a very reduced concentration than would be considered normal) in the ozone layer, around the Antarctic region of the stratosphere, with as high as 33% drop from its pre-existing values in 1975. Even though the Antarctica experiences very little human action, the depletion is due to the fact that extremely cold temperatures with sunshine, and the presence of polar stratospheric clouds (PSCs) makes the ozone over Antarctica very sensitive to the chemicals that destroy the ozone. Looking at the gif, red is good, green doesn't look so good but it could hold, whereas, blue is alarming.
Apart from the CFCs and BFCs, there are other atmospheric gases which, although not classified as ODS, their abundance in the stratosphere as a result of human activities, support ozone depletion. For example, Nitrous oxide (N2O) which reacts to form nitrogen oxides, water vapour and reactive hydrogen in the stratosphere. These substances participate in the depletion of the ozone. And then there's Methane (CH4) and CO2 too, all of which are expected to significantly affect future ozone through the combined effect of chemistry, winds, temperature and some other natural phenomenon on the planet. However, we have the Kyoto protocol that was setup in 1992 to monitor the emission of greenhouse gases and non-ODS that could still be harmful to the ozone.

Ways we could prevent further ozone level depletion.
There are a couple of things we could do to protect our protector, and while this isn't a standard list of the whole solutions available, it's still worth mentioning that a few of these steps, if adhered to, could see the standard ozone level rise to normalcy.
Avoid purchasing aerosol products and sprays containing CFCs. Eco-friendly products are always available as alternatives.
Also, we could avoid the use of fire extinguishers that contains a considerable amount of halogenated hydrocarbons.
We could also help by reducing the number of private cars and compressor-using vehicles which create smog that aids ozone depletion. we could easily take a bus to work, cycle or take a walk.
Enforcement of more stringent rules towards the indiscriminate use of pesticides and nitrous oxide.
Regulations of rocket launches by space agencies, which is the nearest thing to chlorine atoms as far as ozone depletion go.
I believe that in our homes and offices, we really could make a difference, no matter how small, it could make much sense on a global scale. if these tips are followed by everyone, we could get our ozone intact again in a space of fifty (50) years.
Let's protect the Sky, which in turn, protects us!
Thank you for reading,
For the future!
@pangoli
References:


Being A SteemStem Member
yes please and thank you space nerd here
I have a: #SincechannelDegreeInAstrophysics
thanks @pangoli for the post/knowledge
:: :: :: :: :: :: :: :: ::
#sincechannel
#astronomy
#science
#physics
#spacenerd4Life
:: :: :: :: :: :: :: :: ::
<3 #kellyshea
Well done @pangoli! Found your post on Steem-Moderations Discord. Keep it up!
Thank you sir, been a while... i read your post about blockchain abuse earlier
Right on. Much appreciated. Our abuse fighting witnesses need all the help they can get. Please, feel free to dm me your links in the future. People like you are what Steem needs more of and I am glad to support.
Aye Sir!
Thank you
Wow! Your article is really interesting! It is very technical for me, but still easy and fluent to read.
I honeslty think that we, as Earthlings, are very lucky but it's all a matter of balance. Every action has a reaction, even the smallest one. If we have a power, this is our understanding of how Earth works: we can prevent our actions from producing catastrophic reactions, but it is up to us!
The first step is knowledge, so thank you for your post, it helps a lot to understand how fragile our system is and gives us useful tips to start doing something good for our planet!
Congratulations @pangoli! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
To support your work, I also upvoted your post!
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOPVery impactful piece. Indeed no knowledge gained is wasted. Thanks
Very Impactful piece! Indeed, no knowledge is wasted. Thanks
Yeah well constructed article. I love this topic. But then what about gas flaring? Don't you think we need to go green?
I'm a pretty solid green supporter. and from what i know so far, gas flaring increases the concentration of co2, which is a greenhouse gas. bad for the long run of things, Which is why measures are already in place. Nigeria has 2020 set out as deadline for gas flaring. w going green with the rest of the world
well-defined
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by pangoli from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.