And that's why Carbon is a tramp... --- Wait, there's more!

Hey everyone, good to be back again. Haven't written anything outstanding the past two days. well, that's because this one took a whole day, and I thought I should knock myself out.
Anyway, I'm Still searching for that sweet-spot, - the balance between Steemit and schooling-, and somehow, I feel I'm gradually getting it.
So I was watching a show about carbon the other day on TV, and I found a lot of interesting facts about our carbon guy, that I decided I was going to carry out a research on it. It's pretty amazing to discover that so much about nature that we see, know and love, has a lot to do with carbon. It's mind-blowing, and it gets interesting as we delve.
Now here's a thought; Imagine that the most handsome and beautiful person you've ever known is just a string of atoms rumbling in water... You see, it's true, I am made up of atoms, we all are.
Carbon is the fundamental foundation of biology, so much that scientists find it difficult to talk about the formation of life without referring to a page from carbon's book.
In our usual fun-and-engaging-yet-scientific Way, here's why I think carbon is a tramp:

What is Carbon.
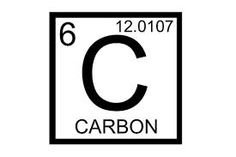
Carbon is an element belonging to the group 4 members on the periodic table. Member elements in group 4 are very well known for having valency (number of outermost electrons) as 4.
Carbon is pretty small, in comparison to many other huge atoms on the periodic table, and it is damn consistent with its numbers too (With 6 protons, 6 neutrons and 6 electrons), the figures align real good to give it an atomic weight of 12.
And because carbon doesn't take much space, it could behave in many different ways. For one, it could easily twist and turn and shape itself, it could flatten out like sheets or spin as in spirals, to attain whatever shape it desires, and could even go as far as forming double and triple bonds with itself and other compounds too. See it as your equivalent Olympic gold gymnast. Carbon can do a lot more things than other bulky atoms cannot afford to do.
When carbon behaves this way (Form long chains with itself, ) we say it forms an Organic compound – Which are basically classes of compounds that contain carbon, regardless of what else they contain, and such a bond is called a Covalent Bond.

Fact: Carbon is kind and gentle. It shares its stuff with others!
An atom? kind? How's that even possible?
Using the word "kindness" on an inanimate " thing," sounds quite interesting and could crack you up a bit. However, with a total number of four (4) electrons orbiting its outer shell, carbon really is kind and gentle. It doesn't need to be brute get what it wants by bullying any of the other atoms or using just any malicious means it could. It isn't as desperate to cast off an electron like Sodium is. Sodium literally explodes when in contact with water, or as vicious as Chlorine looking to get an extra electron, so much that it would tear down the lining of your lungs if you breathe it in. No, Carbon... is calm, (be sure you read that slowly)* and doesn't bear a grudge.
If I were a carbon atom, sure it would be great to keep extra 4 electrons to myself alone, but our carbon knows what it feels like to be lonely. Hence, it prefers to share its valent electrons with some friends. And by golly it has got a good number of them, seeing that it can form bonds with a lot of other atoms.

Fact: Carbon's got strength!

As the 15th most abundant element in the Earth's crust, carbon's atoms bond in different ways to form allotropes. These "allotropes" are basically the same carbon atoms arranged in a different way. Some of the best known alotropes of carbon are Graphite, amorphous carbon, Fullerenes and the unchalleged Diamond!
Diamond as a good offset of carbon, is the hardest naturally occurring substance yet known to humans. Also hard materials such as steel and metal cutting tools contain and such high percentages of carbon! it really is a strong one.

How Carbon does it? The magic of Covalent bonds.
Well, Carbon has a total number of 6 electrons, with two electrons in its inward Shell and four others circling without, as valent electrons. Now, we know that every atom strives to get to a state of stability by attaining either a duple valency or an octet valency. For duple, it's just two electrons on its outer shell, and that pretty much makes it stable (like the Helium). for octet, they require 8 electrons to be stable. These electrons sit in pairs of two, and wouldn't go close to each other until it was up to four.
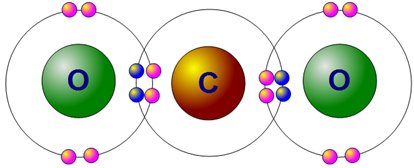
So carbon has four already, and it needs four extra electrons to reach the much coveted state of "happiness and stability". It does something really cool; It looks out for other atoms that also are on the lookout for certain amounts of electrons too, (like our oxygen friends by the left here)and sort of binds and shares these electrons with each other so that everyone wins in the end. As a foodie that I am, I like to think of it as several friends without sufficient money to get a cake each for themselves, so they come together and contribute enough cash to buy a much bigger cake, and everyone gets to share. This allows carbon to form covalent bond with a plethora of atoms such as Hydrogen, Oxygen, phosphorus, Nitrogen, and almost every member of the Halogen (7) group.
Perhaps what is even more special about this carbon guy is the fact that it could do this on his own. – It could form bonds with itself over and over again, and would even form double and triple bonds if it has to.

Thought box: Methane!
Here's how Methane, the smallest member of the organic class of compounds is formed:
Carbon needs 4 electrons, and hydrogen which has a single electron desperately needs to find an extra electron to become stable. So they collude, Hydrogen goes back home and gets three other guys like himself. Then each of them shares an electron with carbon, and voila!, our very popular Methane gas (CH4) happens, and everybody wins!

Carbon could make all of these bonds in an infinite amount of ways, of which some are yet to be discovered. Hence, it's easy to see why it is the core atom of some really complicated structured and systems, such as ourselves!

Fact: Carbon supports life!
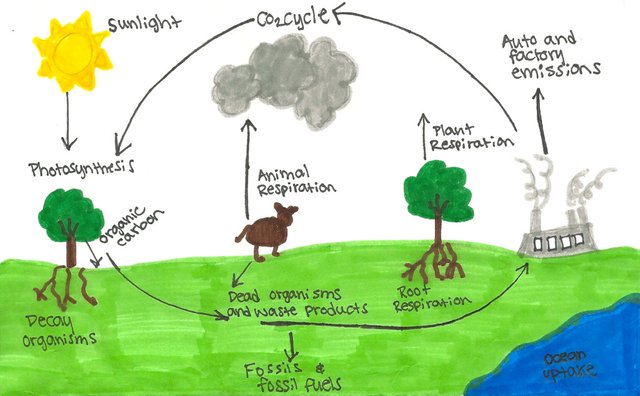
Carbon supports life as it is constantly floating around in the atmosphere as CO2 gas, which is essential for plant growth and production. This is known as The Carbon Cycle, where carbon is constantly being exchanged between the earth's ecosystems – The Biosphere, Atmosphere, Hydrosphere and Geosphere.
Basically plants "breathe" in CO2 and give off oxygen as a byproduct, Whereas, animals take in oxygen and give off CO2. So it's a two way thing. This is one of the reasons why our air isn't saturated with CO2 wastes from humans and cars. As such, it's really a great idea to have a few flowers and trees around as they help purify the air.

So, let's talk a bit on Carbon's class of compounds, Specifically on how they're are represented. Granted, all of this happens on an atomic scale, so how do scientists know what is going on? How do we even interpret that?
Answer: We use a system called the Lewis dot structure!
The Lewis dot structure: Help! What's that on the chalkboard?
Before I got to learn about what it all entailed, I saw these things as some strange characters whenever I passed a senior class during chemistry lectures.
The Lewis dot structure is a very useful tool for chemistry bonds in general, and specifically for easily representing these covalent bonds on paper! It was named after Gilbert N. Lewis, who developed and introduced it in his article, "The Atom and the Molecule" in 1916
The Lewis dot structure follows the octet rule that states that elements with more than one shell would require 8 electrons to fill up its outer shell in order to be stable and happy! Hence, it simply uses dots to denote where these covalent bonds between atoms exists.
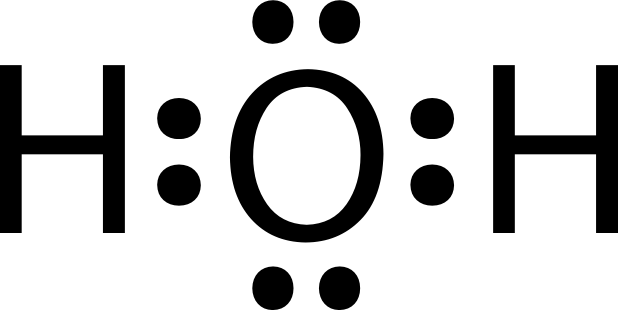
Again, here's a good illustration of other atoms that aren't carbon. Let's get to see how our water is written using the lewis dot structure.
Oxygen with 6 electrons in its outer shell requires 2 extra electrons to be stable and happy. Bringing two atoms of hydrogen folks, a deal is struck, and we get out water.

Thought box: NaCl – Sweet and nasty.
As earlier stated, Sodium and Chlorine exist as rogues on their own. But a lot could change when these guys come together to make a single compound.
It's a give and take thing that occurs here (Not necessarily a covalent bond). Sodium is desperate to give out an electron, and Chlorine is desperate to acquire an electron. So we setup a meeting and bring these guys together to make your regular table salt. – Just like how it would be a good idea to get two crazy friends together, so that they could stop disturbing you.
This type of bond is called an ionic bond or an electrovalent bond, where an atom gives off electrons totally and becomes a charged atom. Well, there's no such thing as a "charged atom", so we call it an Ion.


Fact: Carbon in our daily use.

From applications in back-dating stuff up to millions of years ago, –As in carbon dating–, to basic household accessories we find around, Carbon has found itself neck-deep in shaping our world, and it's advantages and applications are innumerable, in a way.
Below is a short list of some of these uses and applications in no particular order:
In petrochemical plants, carbon is integral to the processes of refining
In our bodies, carbon is essential for growth and strength too. After all, a whooping 18% of our body is made up of carbon.
Some really heat resistant materials are made of carbon. Like metals such as steel which has worldwide applications in many areas.
Plastics – Which are perhaps the most abundantly produced and used material today is made from the polymerization of organic compounds, which of course is made up of carbon.
Carbon is used as CO2 in drinks as a preservative and in some daily use materials such as shoe polish, perfumes, carbon paper, etc.
It also is used in pencils as graphite for writing and is used as charcoal for fuel.
Seeing all of the oddness and weirdness in carbon, it's easy to understand why someone would call it a tramp or why We'd even care!
To me, carbon isn't just a tramp, it's an Emperor!
Thank you for reading,
Let's do this again sometime!
@pangoli

References:
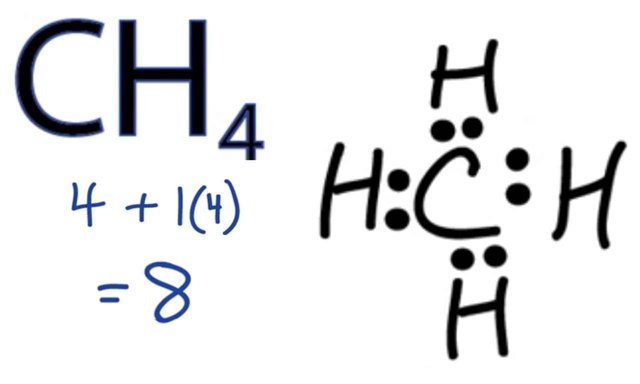

Amazing how you've explained all this in a way that literally anybody who doesn't know about chemistry can understand it. Congratulations !!
Quite interesting, With a mix of humor and your concise writing style, you managed to make basic chemistry fun!
Yup, this was fun writing... it's a whole new approach to learning stuff as complicated as chemistry.
Baby!
@pangoli, burst my brain already.
This is indeed 'proof of brain'.
Your articles are scientifically interesting 😀
Keep it up!
That's such a sweet thing to say Jeline... thank you
How do i cope with this scientific analysis?🙆
"It gets easier with time" – @greenrun
Yes it does!
Lmao.... I had fun reading this. It's quite engaging.
Hope you learnt something cool... Carbon is a tramp! And a darn good one we all need!
Awesome post! Thank you, Pangoli
Wow, beautifully written and outlined.
I love the way you explained everybit of it.
If only my teachers explained my elementary chemistry, who knows?. I might become a chemist😁😁
Being A SteemStem Member
Carbon plays a fundamental role in all life and everybody uses it everyday. Carbon is a tramp!
I can see why it took you the whole day; you did a great job. Thanks for this bro @pangoli