Understanding The Electronic Configuration That Dictates The Conductivity Of Metals
A good understanding of the underlying concepts of current and voltage necessitates a degree of familiarity with the atom and its structure; this is so because atoms are the basic structural unit of all engineering materials.
A recent study has found out all matter be it solid liquid or gaseous, constitutes of tiny particles called molecules. The molecules are made up of minute particles called atoms. Those substances whose molecules are made up of similar atoms are known as elements while those whose molecules consist of different atoms are called compounds.
An atom is the smallest particle of an element that retains the characteristics of that element. It consists primarily of three basic subatomic particles: electrons, protons and neutrons. Each atom of an element differs from the atom of the other element, and this gives each element its unique characteristics (atomic structure).Different atoms comprise of various numbers of electrons in their concentric shells surrounding the nucleus.
The first shell, which is nearest to the nucleus can only accommodate two electrons.If an atom should have three electrons, the third electron must go to the next shell.The second shell can only contain a maximum number of 8 electrons, the third 18, the fourth 32 as determined by the equation 2n2 where n is the shell number. These shells are usually represented by numbers: (n=1, 2, 3, 4….) or letter: (n=k, l,m, n…….).
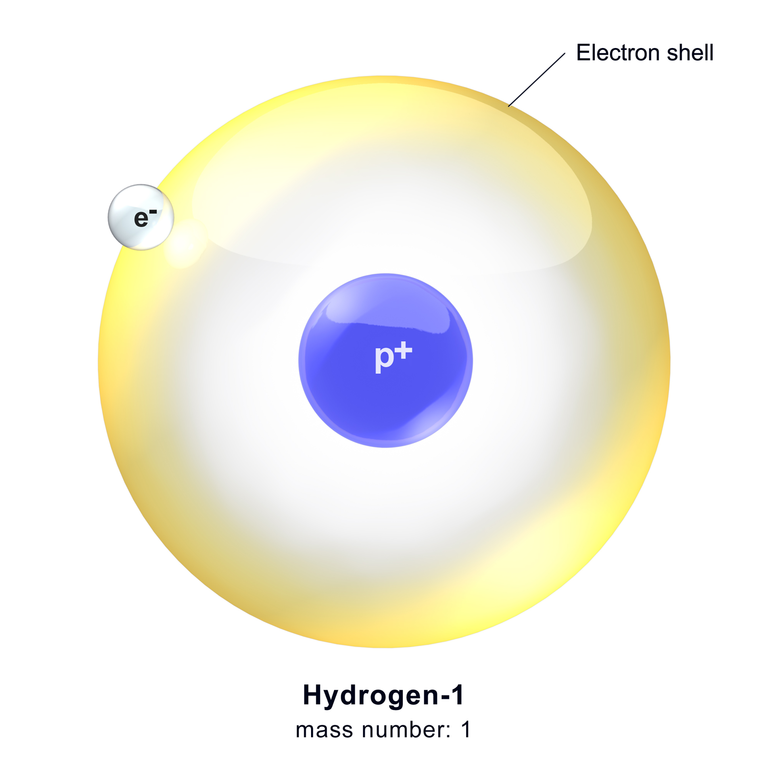
Wikipedia: A Hydrogen Atom
According to Bohr model, the atom is made up of a central nucleus surrounded by orbiting electrons. The electron has a relatively small mass of 9.11 × 1028g and a unit charge of -1.602× 10-19C , while the mass of the proton is 1.672× 10-24g and a unit charge of +1.602× 10-19g. The mass of the neutron is slightly heavier than the proton and is 1.675× 10-24g but with no charge.
The electron has a negligible mass when compared to a proton. Its mass is approximately 1/1836 that of a proton. The electrons, especially the outer ones, determine most of the electrical property and a basic knowledge of atomic structure is essential if we fully want to understand the movement of charges.
Each type of atom has a certain number of electrons and protons that differentiates it from the atoms of all other elements. For instance, the simplest atom is that of hydrogen which has one proton and one electron as depicted below. Another simplified example is that of helium atom which has two protons and two neutrons orbiting the nucleus.
Atoms of all substances consist of identical protons, neutrons and electrons, etc., the difference lies in their number and its configuration. It has been established that the positive charge of the electron is numerically equal to the negative charge of an electron. Naturally, an atom is electrically neutral because the number of electrons equates that of the proton.
The arrangement of elements in the periodic table is based on their atomic number. The atomic number equals the number of protons in the nucleus, which is the same as the number of electrons in an electrically stable atom. For example, hydrogen has an atomic number of 1 and helium has an atomic number of 2. In their neutral state, all atom of a given element possess the same number of electrons as protons; the positive charge counteracts the negative charges, and the atom has a net charge of zero.
Electron orbit the nucleus of an atom at certain distances from the nucleus. Electrons near the nucleus have less energy than those in the more distant orbits.
Energy levels - Each discrete distance (orbit) from the nucleus corresponds to a specific energy level. In an atom, we group the orbits into energy bands known as Shells. A particular atom has a stipulated number of shells. Each shell has a fixed maximum number of electrons at permissible energy levels known as orbits.
Electrons that are situated in orbits farther from the nucleus exhibit higher energy and are less tightly bonded to the atom than those closer to the nucleus. This bond is due to the force of attraction between the positively charged nucleus, and the negatively charged electrons diminish with increasing distance from the nucleus. Electrons with the highest energy exist in the outermost shell of an atom and are relatively loosely bound to the parent atom.
This outermost shell is known as the valence shell, and therefore we refer to it as valence electrons.These valence electrons influence the chemical reactions and bonding within the structure of a material and dictate its electrical properties. The term valence signifies that the potential needed to dislodge any one of these electrons from the atomic structure is much lower than that needed for any other electron in the structure.
When an atom absorbs energy from a heat source or sunlight or ordinary incandescent lamp, the electrons are excited (the energies are raised). The valence electron possesses more energy and are less tightly bound to the atom as compared to the inner electrons and thus can quickly jump to the higher orbit within the valence shell when they absorb external energy.
If a valence electron acquires a sufficient amount of energy, it can easily escape from the outer shell and the atom's influence. When the valence electron leaves a neutral atom, it impacts excess of positive charge (more protons and electrons). The process of losing a valence electron is known as ionisation, and the end product is a positively charged atom called a positive ion. For instance, the chemical symbol for hydrogen is H.
When a neutral hydrogen atom loses its valence electron and becomes a positive ion, we designate it as H+. The escaped valence electron is called free electrons. When free electrons lose energy and fall into the outer shell of a neutral hydrogen atom, the atom becomes negatively charged (more electrons than protons) and is called a negative ion, designated H.
If an atom should have three electrons, the third electron must go to the next shell. The second shell can only contain a maximum number of 8 electrons, the third 18, the fourth 32 as determined by the equation 2n2 where n is the shell number. These shells are usually represented by numbers: (n=1, 2, 3, 4….) or letter: (n=k, l,m, n…….)
The maximum number of electrons (Ne) that can exist in each cell of an atom is a design of nature, and we can calculate it with the formula below:
Ne = 2n2
Where n = number of the shell under consideration. The innermost shell number is 1 (from the centre), and the next shell is two, and it continues like that to the outermost shell.
For the innermost shell designated as shell number 1;
Ne = 2n2 = 2× (12) = 2
The maximum number of electrons that can exist in the second shell is
Ne = 2n2 = 2×(22) = 2x4 = 8.
The maximum number of electrons that can exist in the third shell is
Ne = 2n2 = 2× (32) = 2×9 = 18
The maximum number of electrons that can exist in the fourth shell is
Ne = 2n2 = 2× (42)= 2×16 = 32
The force of attraction or repulsion between two charged bodies Q1 and Q2 was determined experimentally by Coulombs to be (KQ1 Q2)/r where F is the force in Newton, K=constant=9×109, Q1 and Q2 are the charges in Coulombs and r is the distance in meters separating the two charges.
An experiment by Coulombs proved that unlike charges attract (charges with opposite polarity) and like charges repel (charges of the same polarity). Based on the above conclusion, it, therefore, means that electron will repel each other and proton and electrons will attract each other. But we also know that the nucleus contains protons which are positively charged which means that there will be an attractive force between the nucleus and electrons in the innermost orbits (smallest r).
As the distance from the nucleus and the orbital electrons increases, the binding force weakens reaching its lowest at the electrons in the outermost subshell (largest r). As a result of the weaker binding forces, less energy will be able to dislodge an electron from an outer subshell.
We can easily remove electrons from atoms having incomplete outer subshells and possessing few electrons.
This characteristic of the atom that that allows the removal of electron under specific conditions are crucial if motion of electron is to be initiated.
We will examine the electronic configuration of aluminium, copper and silver to drive home the point. Copper is the most widely used metal in the electrical and electronic industry owing to it excellent conductivity and cost advantage as compared to silver.
Looking at the electronic configuration of copper, you will notice that it has an extra electron in excess of what is needed to complete the first three shells. The incomplete outermost subshell, having only one electron, coupled with the distance (largest r) between the electron and the nucleus, shows that the twenty-ninth electron is not tightly held to the copper atom.
All it needs to break away from the parent atom and become a free electron is to gather enough energy from the surrounding medium.The term ''free'' is applied to an electron that has left the fixed lattice structure and very sensitive to any applied electric fields which can come from voltages sources or any difference in potential.
These free electrons move about in a randomised form of motion, and the net movement in any one direction is zero. But, with a little application of potential difference in the form of a battery, there will be a flow of electron in the preferred direction of the voltage.
To fully appreciate why silver, copper and aluminium are the conductors of choice in the electrical and electronic industry, requires some understanding of the electronic configuration. They all possess one valence electron in their outermost shell which can easily be excited to leave the lattice structure to become free electron.
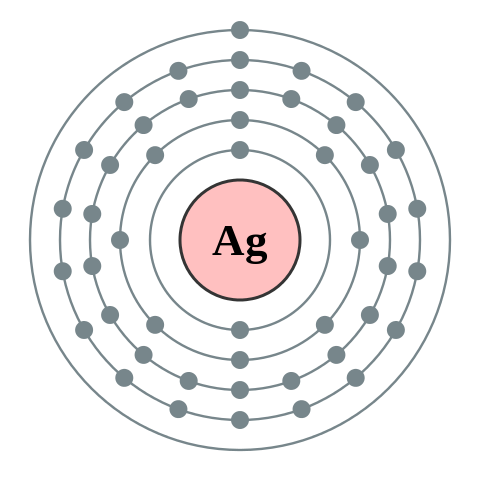
Consider the following diagrams showing the electronic configuration of silver, copper and aluminium with their position of valence electrons which is very far from the nucleus and away from the attractive forces that could have prevented them from breaking away.
Silver has atomic number of 47
1st shell: 2n2 = 2× (1 2) = 2
2nd shell: 2n2 = 2× (2 2) = 2(4) = 8
3rd shell: 2n2 = 2× (3 2)= 2×9 = 18
4th shell: 19 electrons
Copper has atomic number of 29
1st shell: 2n2 = 2× (1 2) = 2
2nd shell: 2n2 = 2× (22) = 2(4) = 8
3rd shell: 2n2 = 2×(3 2)= 2×9 = 18
4th shell is left with one electron.
Aluminum has atomic number of 13
1st shell: 2n2 = 2× 12 = 2
2nd shell: 2n2 = 2× (22) = 2(4) = 8
3rd shell is left with three electrons
CONCLUSION
It is easy to see that the fourth subshell (f) of the silver atom has only one electron where it can take a maximum of 14 and as such the single electron can easily break away from the parent atom and be free for conduction. Looking at the copper atom, it is clear that the fourth shell has only one electron which is quite away from the attractive forces of the nucleus, thus can easily break away even at room temperature for conduction. The same line of reasoning goes for the aluminium which has three electrons in its outermost shell. From the second subshell of the outermost shell, notice that it has one electron where it is supposed to have a maximum of six. Hence it can be easily removed from the lattice structure for conduction.
Electricity involves the flow of electron, and if there is no free electron, there will be no flow of charge or current electricity.
_enhanced_Bohr_model.png](https://steemitimages.com/DQmWaupYF2idC4D4VdBYd9ptDweBVp9FgLWRh1mTJcKdyPA/29_copper_(Cu)_enhanced_Bohr_model.png))
_Bohr_model.png](https://steemitimages.com/DQmRKuiiX13c6JLzqt7H2PxDVLUJbi2wufdBeCsfYjW5K9M/13_aluminum_(Al)_Bohr_model.png))
)
_1[2].jpg](https://steemitimages.com/DQmYMKsKxxpDtKekejeMdPW29hoTghfAVC92Zp9Tk3ri81m/New%20Doc%202018-01-18%20(2)_1%5B2%5D.jpg))
_3.jpg](https://steemitimages.com/DQmf2tjHk58uKSdttz2VE1eXdZwaR2AL7jUePyAtVMg1C9N/New%20Doc%202018-01-18%20(2)_3.jpg))
_2.jpg](https://steemitimages.com/DQmRj3kSxtmM8miiqMdFxKNCxaQvvjpxW1vjJ7GueyDdv3v/New%20Doc%202018-01-18%20(2)_2.jpg))
your post is very good, i love to read it. I will be your fans, because all your posts are very interesting for me to read.
best for you @kaydee
Thanks for stopping by and your patience to go through the details of the post is really appreciated. The electronic configuration is the bedrock of properties of Engineering material. But in this case, it was restricted to conduction and current electricity. I am your fan too, that I assure you.
Very interesting!!!!
I am happy it made sense to you. Thanks for being there.
Any time!!!!
Great post man. I love to read Chemistry. This is a great chemistry post about proton. This is my post of acid-base:-
https://steemit.com/science/@pradeeprajora/theory-s-about-acid-base
Thanks for appreciating the work. I will look up your post immediately. Chemistry is quite vast but interesting.Thanks for stopping by to read the post.
It made me feel so nostalgic reading this post as I learn Chemistry in my schooldays. Very informative article. Upvoted!
Many thanks. You can never do away with chemistry.Very important course. Thanks once again for appreciating the post.
Great post kaydee! i love learning about electron orbitals. You are correct having that single valence electron is very important. I have a theory that free radicles in our body produced naturally through metabolism are what cause various diseases and thats why you hear about products like colloidal silver. A kind of electron dump that soaks up loose free radicles.
Thanks for your great understanding of the post. I really appreciate your theory too about free radicle. Maybe in the future, you will receive recognition for your diligent works.