Theory's about "Acid-Base".
Hello steemians friend's, my exam of Inorganic Chemistry will come on next Saturday. I have some preparations of exam for the topic "Acid - Base ". Today I want to talk about It.
In this lesson, we discuss about quality of acid - base by these principles.
- Arrhenius Theory
- Bronsted - Lowry Theory
- Lux -Flood Theory
- Solvent - System Theory
- Lewis A - B Theory
Now we discuss about these Theory :-
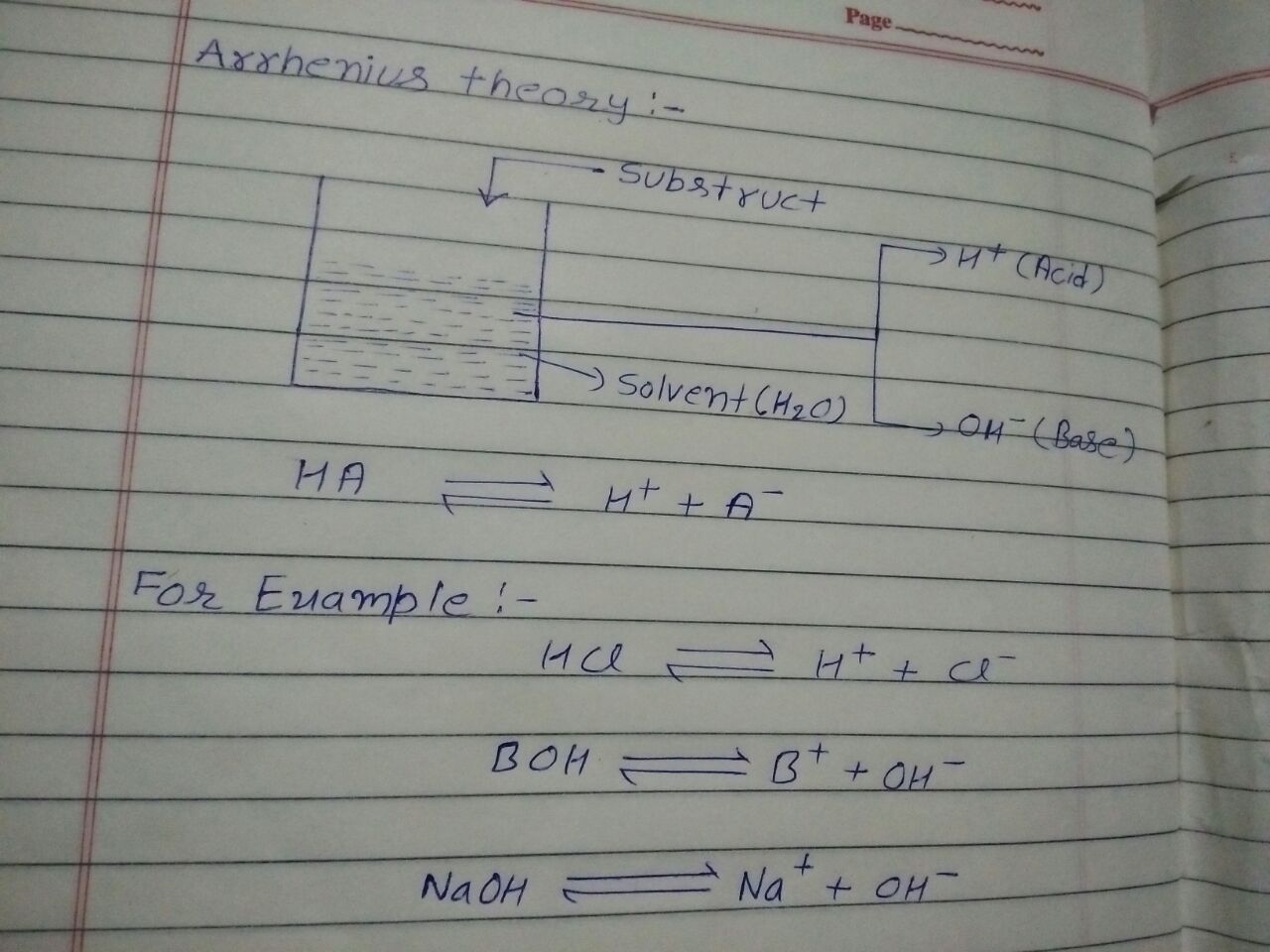
1. Arrhenius Theory :- This principle added Arrhenius in 1884. In this theory "when we dissolve any Solvent in water and it gives hydrogen ion that it calls acid and when it gives hydrocsil ion that it calls ".
Sorry for my bad handwriting!!!
Uses of Arrhenius Theory :-
- According to this theory for all of strong acid and strong base are always constant in neutral .
- In this theory acid and base are affordable.
- In this theory it discribe about catalyst of acid by hydrogen .
Disuses of Arrhenius Theory :-
- Some of speaces who not give hydrogen ion are acids.
- Gain of hydrogen ion are not include acids.
2. Bronsted - Lowry Theory:- This principle lunched 1923 by Bronsted and Lowry. According to this theory Solvent who gives protons are called acid and Solvent who takes protons are called base.
Uses of Bronsted-Lowry theory:-
- From this theory we know about many base's .
- This theory say's about sodium carbonet is a base.
- According to this theory it fixed feric cloraid is a acid.
Disuses of bronsted lowry theory :-
- Many of acid-base reaction aren't have a single .
3. Lux-Flood theory :- It called oxide ion concept and it launched on 1939. In this theory solvent who takes oxide ion are called acid and solvent who give oxide ion are called base.
Uses of Lux-Flood theory:-
- Oxide of bases are negitive in there alfa numbers.
- Oxide of amphoteric are near about zero(0) in alfa numbers.
- Oxide of acid are positive in alfa numbers.
Disuses of Lux-Flood Theory:-
- Solvent who haven't Oxide ion are not define by this theory.
4. Solvent-System Theory:- According to this theory acids are these things who Increase positivity of ions and base are dicrease positivity of ion.
Disuses of Solvent-System Theory:-
- This theory can't dicribe about physically uses are things.
- Many reactions are acid - base, it can't .
5. Lewis A-B theory :- According to the principal things who gain electron are acids and who gives electron are called .
It's my lesson "Acid-Base" in Inorganic chemistry, Unit-5. I'm ready to face exam. Thanks for learn it with me.




Thanks for directing me to here. Most of them are definition and could be found in the internet, but there are several things I dont really understand what you mean, like
"...... discuss about quality of acid - base"
"Arrhenius Theory....... when we dissolve any Solvent in water and it gives hydrogen ion "
"..... acid and base are affordable. "
"discribe about catalyst of acid by hydrogen"
"Some of speaces who not give hydrogen ion are acids. "
There are way more spelling mistakes and can you just read once or check all your spelling with some software before putting them here?
I am not going to check every single details here for you.
If you want to get a better upvote for your science article, be sure to check the suggestions around, like this.
All the best with your exam ~
Thanks dear to suggest me for my mistakes. I'll carefully next time. I was convert Hindi to English and make some mistakes. Thanks for your best wishes for my exam.
Awesome
Thanks for learn Acid-Base with me @aks0
My love for Chemistry is undying because I Iike following reactions process by process till the final result. Quite a clear presentation. Thanks for sharing.
Thanks @kaydee for reading my post.