The Seebeck Effect: How to generate electricity from heat using two coins
Just as you can generate electricity from solar panels in the sunlight or from an antenna in the presence of microwaves, you can extract energy from the flow of heat energy. You can do this with pre-made modules like thermocouples or thermoelectric elements. Or, as I will show you here, you can just use two coins (I used a US quarter and penny) to generate a little bit of electrical current and voltage off of nothing but heat.
Here is my setup. This produces a tiny, tiny amount of electrical current (electricity) when you heat up the penny. I'll go over this is much greater detail below.
This experiment is totally safe to reproduce, provided you don't burn down your house.
First, some background on what is going on.
The Seebeck Effect
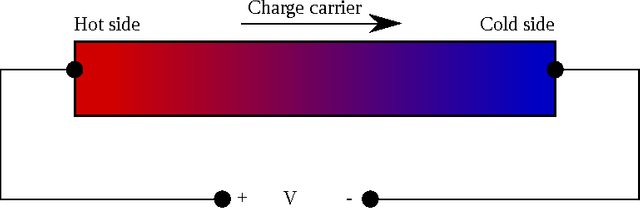
It's first important to note that heat is NOT how hot something is. This seems like a silly distinction but it makes a difference here. A pot of boiling water has a high temperature, and has a lot of internal thermal energy contained within in, but (neglecting heat transfer through the stove and air) there is no flow of heat. If I pour a bunch of cold water in to the boiling water, there is now a flow of heat from the hot water into the cold water as the two mix. This is why touching metal in an otherwise comfortable room feels cold: The metal is more thermally conductive than the air, so heat can easily flow from you into the chair and cool you down, causing you to feel chilly.
With that in mind, something called the Seebeck Effect allows us to extract electrical energy using transfer of heat. Take two different metals and connect them at a junction. If there is a difference in temperature across the junction between the two metals, electrical current will flow, given by a voltage given by:
In this case, EMF is the produced voltage across the junction. Voltages produce currents, so this temperature difference will produce and dissipate electrical energy. The S variable is known as the Seebeck Coefficient, and depends on the materials used.
This is a big deal - it means that we can actually generate electricity just off of heat moving from one object to another. Since the laws of thermodynamics state that there is a statistically overwhelming probability of heat flowing from a hot object to a cold object, we need to generate a temperature gradient (basically just two areas with different temperatures) to cause heat to flow and to generate electrical current.
This is the principle behind thermocouple temperature sensors and thermoelectric generator modules. If you've ever seen one of those gimmicky USB mini fridges, you have seen a thermoelectric module (although in the case of the fridge, it's being run in reverse - put in electrical current, get out the temperature gradient).
Peltier Modules like this one can generate electricity using the Seebeck Effect.
Credit
I actually have a Peltier module on my desk right now, but just using that to demonstrate the Seebeck Effect is frankly kind of boring, especially since we can't easily see the inside. Instead, I succeeded in making a very inefficient thermoelectric generator using nothing but two coins.
Making a thermoelectric generator with $0.26 in coins
As seen above, all we need to do to produce some sort of voltage difference is to connect two different types of metal and heat up one side. This will produce a temperature gradient and therefore, by the Seebeck Effect, a voltage difference and the corresponding electric field.
I just tried to do this with a kitchen knife and was disappointed when it started burning (I'm not sure what kind of crappy materials are in this "stainless steel" knife). It also produced no detectable voltage (thankfully the burnt parts wiped off easily in the sink). While trying to think of a better way to do this, I saw a pile of spare change on my desk.
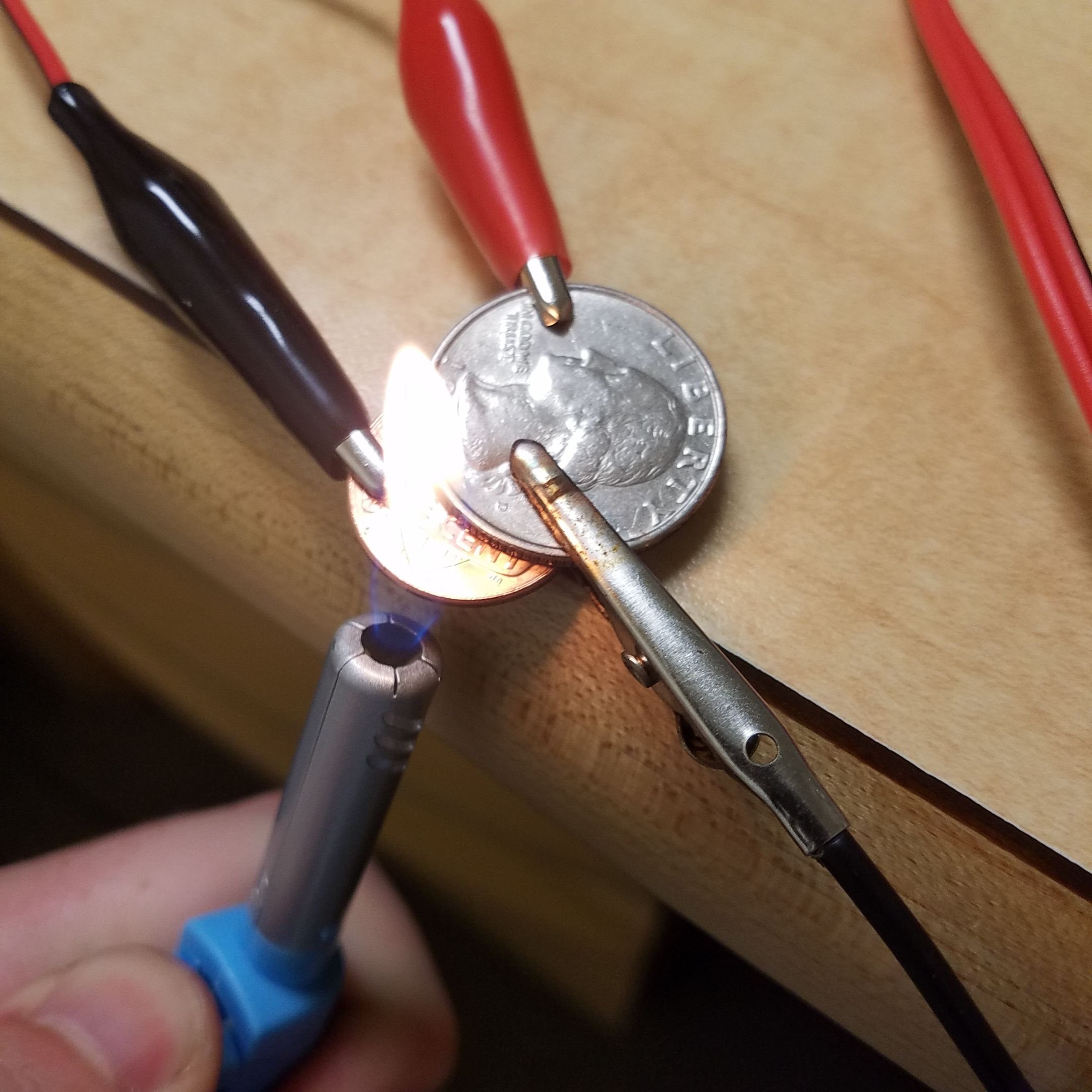
I used a new 2017 American penny (which is coated in an extremely thin layer of copper (Cu)) and a 1991 quarter (which is coated in nickel (Ni)). An old alligator clip that was burnt in a high voltage experiment gone wrong serves as a clamp to make sure that the quarter and penny stay connected to each other.
And... that's it. This is now a functioning thermoelectric generator. Yeah, it really is just two coins clamped together - that's the beauty of the Seebeck Effect. I connected one lead from my multimeter to the quarter and one to the penny to measure voltage changes.
At first, you don't see anything at all - the voltage and current both read zero. This is because there is no temperature gradient - both coins are at room temperature. If you pinch one of the coins, you will heat it up, produce a temperature gradient, and cause current to flow through the multimeter. However, due to the inefficiency of this simple, crude device, you probably won't be able to detect this tiny current, especially not on my $10 multimeter. So I went with the next best thing and used my piezoelectric dollar store lighter, pictured above the coin generator (see this post for how the lighter works, as I think it's a pretty fascinating device in itself)
To generate detectable electrical energy, just light up the lighter under the penny. The flame quickly heats the penny, but as it is somewhat localized, the quarter does not heat up nearly as much. Also, copper is more thermally conductive than nickel, so the penny really heats up faster than the quarter.
Since the rusty burnt middle clamp is holding the quarter in contact with the penny, we now have a significant temperature gradient across a junction of two different conductors: The Seebeck Effect does its work.
The voltage produced is very small, but it is detectable. Interestingly, it takes a long time for the heat to transfer from the penny into the quarter, which means that the small voltages produced actually last awhile (it takes a minute or two for the voltage to drop to zero on the multimeter!). This makes the coin generator a somewhat cool way to visualize the speed of heat flow without an expensive infrared camera.

Here's the voltage produced across the device after heating up the penny for 5 seconds with the shown lighter. Yup, that's a "massive" 0.006 volts - 0.6 millivolts! Not very useful of course, but theoretically you could string these devices together to boost the voltage, or just heat up the penny for longer. As mentioned before, this voltage takes quite a while to decay back to zero due to the finite speed of heat transfer between the coins. Even if the voltage produced is small, it is undoubtedly due to the Seebeck effect and the flow of heat.

And here's the current flowing across the multimeter, generated by the coins. That 4 microamps, or 0.000004 amps, which is actually more impressive to me than the 0.6 millivolts of potential.
At 0.006 Volts and 0.000004 Amps we are producing a massive 24 nanowatts of power (which is hilariously actually really close to the power produced by my Tritium nuclear battery for way, way less work... although the nuclear battery power is more usable as it is at a higher voltage) by heating up a penny! As you can see this isn't really practical in its current form, but I'm sure there's a way to boost the efficiency (such as, not using random pennies and quarters...). Remember that different materials have different Seebeck coefficients and may work better. The coins will pump out this power for around a minute after being heated, with the power value decaying over time.
So, the morale of the story is, if you want to generate electricity from heat, buy a Peltier module and don't use two coins. But, if you want to go mess around with this effect, it's extremely easy - theoretically you could do it with just $0.06 in USA currency (or way less if you use another country's coins). Just make sure the coins are made out of different metals and you are good to go! I had fun doing this because it was such an easy but dramatic way to demonstrate a basic physical law.
Is there a way to make multi-coin thermal generators practical? Probably not, but I will almost certainly be messing around with this more in the future and trying to make it more efficient.
One last quick note: This actually works with just one conductor as well - heat up one side and current will flow across the conductor. In this way I was able to get an even tinier voltage by heating one side of the quarter. Since this is way less effective than going the two-conductor route, I haven't covered it extensively in this post, but it does work if you want to give it a go yourself!
See? There is some reason to use physical coins over Bitcoin...
The Seebeck Effect in its simplest form!
Credit
I hope you enjoyed this post and learned something about the Seebeck Effect! Please don't hesitate to let me know if you have questions, comments, or corrections.
If you've got a multimeter on you, I highly recommend trying this for yourself! Let me know of your success.
Thanks for reading!
All images not credited are my own. You are welcome to use them with credit.

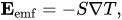

I saw this before but never know it works it in this way!!!
Nice!!
I enjoyed this topic very much. I’m curious if the thermal gradient and varying metals will change the output power.
Different metals do indeed change the output! Each metal type has a different Seebeck Coefficient which then gives how the metal will respond to the temperature gradient.
Interesting post, will have to try this. However, our - Australian - coins are all mostly Cu, with the $1 and $2 coins being 95% Cu and the smaller denominations being 75% Cu / 25% Ni. Not sure how much of an effect will be visible?
In order to see the effect, the external coating on the two coins needs to be different. If the partial nickel coin has the nickel on the outside then you are good to go. I think that one coin being solid copper and one being solid nickel would help, but all you need to observe the effect is two different coated coins. Best of luck if you try it out!
simple science and we have electricity, who said science cant be fun
This is an interesting article. I'm just imagining if this concept can be combined with sun's heat energy to produce some reasonable productivity. Metal that we use will also matter. Not sure how big the apparatus will become to get an efficient output that can actually be useful to us. (By the way, I live in a place where sun fries us all with heat during summer. :))
Remember that this is based on heat transfer! You could absolutely harvest energy using this thing from the sun, but you would need a way to keep the other end of the device cold so that heat could continuously flow from one side to the other. Perhaps a dark coating on one side and a reflective coating on the other would cause more heat to flow from the dark side to the reflective side?
Let me know if you try it!
will this solve the problem of pollution in near future
Definitely not - you still need a source of external energy to produce the heat. In this example that energy source was the lighter.