My DIY nuclear battery: A (more detailed) writeup
Hello everyone.
I recently wrote a post about my nuclear-powered calculator, which was powered by a small, simple tritium nuclear battery I made. I'd now like to go into much greater detail (maybe, too much detail...) on how I made the battery itself, the steps leading to the final design, and how anyone else can build their own for a little under $50.
Unfortunately, I'm away for winter break right now, so I can't post recent pictures of the battery or my equipment/testing setup, but I do have some pictures from my previous Hackaday.io post on this device which I will be including.
What is a nuclear battery?
A nuclear battery is any device that obtains energy from nuclear processes without artificially initiating nuclear reactions. Nuclear batteries are found on almost all spacecraft that travel past Jupiter, including Voyager and the New Horizons Pluto mission. They were also used to power pacemakers in the past, as well as remote lighthouses and sensing stations. If you need power for a very long time, nuclear batteries work well, since the half-lives on many of the isotopes used run into the decades. Basically, you take a radioactive element and extract energy from the produced radiation.
For this project, I used a legal and somewhat inexpensive (~$35) Tritium light source to produce the energy for the battery. The final power output was on the order of 100 nanowatts. Everything on how it was done is detailed below. The rev1 nuclear battery from last year (before redoing it for the calculator) is shown below:

(Image credit: Me)
Why Tritium?
In the US, you can obtain many small radioactive sources legally without a license. Unfortunately, due to the damaging effects of radiation, almost any source that is putting out enough ionizing radiation energy to be useful to a battery would be very very dangerous. That's why tritium is the perfect material for DIY nuclear batteries, as I'll detail below.
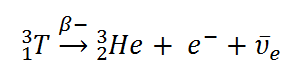
(Image credit: http://www.nuclear-power.net/glossary/tritium/)
Tritium is a radioactive heavy hydrogen isotope that decays with a half-life of just over 12 years. When a tritium nucleus decays, it releases a Helium-3 nucleus, an electron, and an electron antineutrino, of which the electron and antineutrino have the most energy. The antineutrino doesn't interact with, well, anything, and almost certainly will fly off, through the entire Earth, into interstellar space. Since the decay is a 3-body process, the electron (beta) radiation emitted will have varying energy, but on average, the electron's kinetic energy is about 5.7 keV (5,700 electronvolts). While 5.7 keV is well into the ionizing range, for beta radiation this energy is very very small. As such, the beta radiation as well as any secondary radiation (which would have a maximum energy of the highest beta energy, or around 18 keV) is unable to escape the tube in meaningful quantities. Because of this, the tube doesn't release any harmful radiation and will not register on a geiger counter (which I can confirm from personal experience).
The short of it is this: By using tritium to produce visible (in this case, green) light, you can output magnitudes more energy than other radioisotopes at a tiny fraction of the radiation danger (in this, no danger at all).
This means that, out of all of the easy to obtain legal radioisotopes, tritium is by far the best option for building homemade nuclear power sources. For example, an easy to obtain source is Americium-241. You can buy a smoke alarm for a couple bucks online which contains 37 kBq of the material (1 Bq = 1 decay per second, so 37 kBq = 37,000 decays per second), which will then produce 37,000 alpha particles per second. Even though the Am-241 alphas have around 5.5 MeV of energy each, the total energy output still pales compared to a tiny tritium tube. I used a 2 cm long tube purchased on Ebay for around $30, which are usually used as permanent light sources for finding things in the dark. By calculating the gas volume and comparing it to a larger tube online with a known amount of Tritium, I estimate that my tube contaned at purchasing time about 3 GBq of Tritium (or 3,000,000,000 decays per second). Quickly doing the math shows that the tritium tube is producing several orders of magnitude more energy every second than the Am-241 source is, without producing any of the detectable gamma radiation the Am-241 source outputs.

(Image credit: https://roninmetalwerks.com/pages/tritium-info)
Conclusion: Tritium is the clear winner for DIY nuclear batteries. It's legal, it's somewhat cheap, and it outputs a good amount of energy, considering.
How is the radiation kinetic energy converted into electrical energy?
Spacecraft nuclear batteries use large quantities of plutonium to heat up thermocouples and produce electrical energy. This isn't remotely feasible for any small-scale nuclear batteries, since the material will lose heat at too high of a rate to gain any significant amount of temperature due to the high surface-area to mass ratio. Another option, called beta/alphavoltaics, is to directly fire radiation at a PN junction (found in transistors, LEDs, and solar cells) to produce a voltage difference. The trouble with this is that radiation degrades PN junctions over time, and you have to directly expose the radiation source in order to do this, which is not an option when dealing with radioactive gaseous hydrogen.
The solution? Using the light directly off of the tritium to produce electricity the same way solar cells produce electricity from the sun's light (I call this betaphotovoltaics, but I can't remember if I read that somewhere or made it up so take the term with a grain of salt). Now the problem is getting enough light to produce a meaningful voltage on the cell: The tritium light isn't even visible in sunlight, and you have to go into a dark room to see the dim glow.
Here's a picture of my tube under standard lighting:

(Image credit: Me)
Building the battery
After the long wait to receive my tritium tube from an Amazon seller in China, I started testing various solar cells. My first attempt was pretty much doomed to fail, as I tried to get usable voltage off of a 1/2 Watt, 7cm square solar panel (this was all I had at the time). This did not produce a successful device: After completely encasing the panel in aluminum foil to block all ambient light, I was able to measure... nothing. But I was not deterred, because my cheap multimeter probably couldn't pick up such low currents. I did a lot of further testing by charging up 1uF electrolytic capacitors for long periods of time. I was able to pick up a voltage difference of about 1.5 mV (That's 0.0015 Volts!!) after using control capacitors not connected to anything accounting for passive charging (I have no idea why this happens, but if you leave electrolytics out they will slowly build up a few millivolts of potential). 1.5 mV is totally useless, so I wasn't content to leave the device in its current state and call it.
After a lot more testing, I decided to try a smaller solar panel and hope that, since the tritium light would cover a larger portion of the cell, the overall efficiency would be improved and I would produce something detectable. I didn't have a car at the time so I bought a cheap solar powered calculator at my campus' bookstore and gutted it to remove the solar panel.
(Image credit: http://www.makify.com/solar-cell-light-meter/)
I wrapped up the tritium cell subassembly in a bit of aluminum foil for reflecting back lost light and sealed the entire assembly in an mint tin with a 9V battery clips coming out of two drilled holes in the tine. I measured a whopping ... 0.4 Volts. Progress, but still not very usable. However, after my standard capacitor charging test, I found that the capacitor actually charged to 1.6 Volts - usable! Turns out, the cheap multimeter indeed could not properly register nanoamps of current. When I made this 1.5 years ago, I didn't (and still don't) have a nanoampmeter, so I approximated the current by measuring the time it took for a capacitor of known capacitance to charge to the full 1.6V and approximated the total power output based on the energy gained by the capacitor. The conclusion: This DIY nuclear battery produced just around 100 nanowatts, which is terrible and tiny, but USABLE, which was my original intent. Funnily enough, I found an absurdly expensive microwatt level commercial Tritium battery online, and my device actually wins in terms of nanowatt per cost.
At this point, I began looking for ways to demonstrate a use for the battery.
The result: A (really bad) nuclear powered flashlight
If you've ever heard of a joule thief circuit, you'll know where this is going. 1.6 volts, even when the energy is first stored in a capacitor, isn't enough to light a visible light LED due to the required voltage drop (Red LEDs drop out at around 1.8 volts). I found an unlikely solution in, of course, the dollar store.
My local dollar store sells a bunch of solar powered garden light for $1 ($0.25 after holidays when they dump the themed ones!). For that dollar, you actually get a lot: A small crystalline solar cell, a NiCd AAA battery, and the lighting circuit. As the battery was only 1.2 volts, I looked into the circuit after buying some of these lights. The circuit boosted the input voltage up to around 3 or 4 volts and light up a somewhat bright white LED, and could run the circuit all the way down to around 0.9 or 0.8 volts on the battery input. This is exactly what I needed. It turns out that the circuit was using the solar cell as a light sensor and cutting out the light when a transistor-like device picked up positive voltage across two pins from the panel. I cut off the panel, which permanently opened the switch and kept the light on.
Now for the final test: Can the nuclear battery do something useful?
I used the battery to charge up a large electrolytic capacitor to the maximum voltage of around 1.6 volts, hooked it up to the salvaged circuit, and ... the light came on! For a second. But it worked, and I had one of the few nuclear-powered flashlights (even if it was totally unusable as an actual flashlight).
The final result: A nuclear tritium battery, with:
- ~15 of usable lifetime before the light output drops to unreasonably low levels (H-3 halflife of 12 years)
- ~50 nanoamps maximum current output
- ~1.6 Volts maximum voltage
- ~75 nanowatts power output
- NO DETECTABLE IONIZING RADIATION EXITING THE TUBE (you might be able to pick some tiny X-ray flux with a very sensitive scintillation counter)
A year later (this past summer), I improved the cell by making it smaller and increasing the efficiency (reaching about 1.8 volts on the same tritium tube) by better applying reflecting foil and ditching the tin, then used it to power a calculator (see my previous Steemit post on this), which is without a doubt more useful than the 1-second nuclear flashlight I used to initially test this.
One my eventual project goals is to figure out how to get some sort of voltage out of an Am-241 source as described above. So far I have had no luck, other than picking up a few dozen millivolts on an exposed solar cell pn junction for a few minutes which quickly decayed away as the alphas destroyed the junction. If you have ideas, let me know and I'll credit you if I succeed in making a much crappier version of this project with Americium.
I hope you learned something from this project writeup! Hopefully it wasn't too detailed. If you'd like help building your own nuclear battery (keep it legal...) or have ideas for making it better, comment and I'd be happy to help/discuss. And yes, I know about the popular youtube video in which someone made one of these, because I made mine before that video (and anyone else in a DIY setting, to my knowledge).
Thanks for reading!
My original Hackaday post on this: https://hackaday.io/project/12715-tritium-nuclear-battery-betaphotovoltaic
Tips/Upvotes appreciated!
NAV: NZLuonFWZTRL8mYQ41SwaP7qopSTbgDQ72
BTC: 1MV7pvR9PGzYBMbSpAyRQbMBonJxZyBnrT
VTC: ViuLmSCuyQXLTJKZphKPwmTvFfvjc88Woz
A very interesting post indeed! @originalworks

And a astonishing result. I have a few Tritium tubes as well, mostly in watches. Seeing that the light output is incredibly weak, its amazing that you were able to collect anything measurable from a low quality solar cell (as they use in cheap calculator or garden lights), since they have a effency rate of only 10% or so.
Btw, a while ago I saw a Tritium tactical light for sale. I was significantly brighter than those tubes. Enough to read a map and such things. It looks like that:
and is made by a dutch company http://www.betalight.nl/en/outdoor-tactical/torch.html
The @OriginalWorks bot has determined this post by @proteus-h to be original material and upvoted(1.5%) it!
To call @OriginalWorks, simply reply to any post with @originalworks or !originalworks in your message!
Glad you enjoyed the post! Interestingly, despite the low efficiency the calculator amorphous panel was by far the most efficient choice for tritium cells that I tried, even beating out the similarly-sized garden light panel, which in sunlight would be more efficient. The calculators are built to charge in low-light (indoors) environment, so they end up working quite well for this application.
It's a shame that link doesn't give out the activity of the tritium content, but I suppose they don't want people to know that it's radioactive (the word tritium isn't even mentioned on the page, even though that's definitely what they are using!). Either way, I'm sure it would produce a much better cell.
Well, all their products are based on Tritium ilumination, and they do talk about radioactivity, proper disposal of their products and so on. Being a dutch company, and working for customers like armed forces and NASA, I'm sure they comply to EU regulations on radiation limits and radioactive substances. But no, they dont state the radiation output of the torch, but they say the light output is 1,000 µL. They also dont say anything about the price, but I think it was something like 50 or 60 Euros when I saw it on Amazon (its not there anymore).
Fascinating stuff! You should do an update on this so that it can be reposted! Im sure plenty of folks would be interested
Glad you enjoyed the post! I did rebuild this device in my tritium-powered calculator and got a little bit increased efficiency but that was just due to better foil placement. I don't see a way to significantly improve the battery at this point without adding more expensive tritium tubes, so I don't really have the content currently to post an update.
Thanks for the reply. I'm in the homesteading movement and like to post more technocal stuff...it shouldnt all be about living by candlelight snd grubbing roots out of the ground!
That said, Im looking to post some collections of interesting and appropriate technology in some of thee groups. Do you mind if I include some of yours? ALL credit given to the source, of course.
Of course! Feel free to use anything I post or any of my original images, provided you link back to me.
First off, I really, really hope you work for the government at some level (Even as a contractor) just so maybe you won't be put on any lists or worse haha. That said good job man, I'm no type of physicist and certainly not a nuclear engineer, and I've even heard of people failing trying to build a small scale reactor.
But you did you research if you had to, and made your own battery from an energy source we underutilize. Keep up the great work but please stay safe.
I think a nuclear battery can be built by Nuclear fusion,because fusion is more easily achieved with lightest elements such as hydrogen,nuclear repulsion is easily overcome as nuclei approach each other. The raw materials for fusion are more cheaply and readily available. Like the electrolysis of sea water which is cheap and in plentiful supply.
Unfortunately for us, no one has ever achieved break-even fusion even in the biggest facilities. Maybe one day!
That being said, you can build DIY fusion devices (usually fusors) at home, but they usually cost a few thousands of dollars and produce something like a milliwatt of fusion energy for every kilowatt input, so no net output. It's something I eventually want to do, but just for fusion's sake, as no one will produce any usable electricity with a fusor.
Congratulations @proteus-h, this post is the fifth most rewarded post (based on pending payouts) in the last 12 hours written by a Dust account holder (accounts that hold between 0 and 0.01 Mega Vests). The total number of posts by Dust account holders during this period was 4315 and the total pending payments to posts in this category was $516.44. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
This is pretty solid stuff bro... I'm not all clear on things related to radioactivity. Hated it. It's good to know someone out there is being productive with it. Upvoted the little I could.
Nice post.. Awesome
Now that's called optimization (kinda)
this is awesome, this could last a long time and could be useful for small applications like survival torchlight for underwater usage.
like the one in the movie "sanctum"
greate stuff loved the post :)
excellent post, greetings and I'll follow you
haha nice job!
I had been playing with Am-241 sometime ago
Personally I just feel like it is a bit like "cheating"(haha) if use those tritium tube. I had been thinking if possible to directly convert the radioactivity directly to electrical energy but seems not that trivial.
Anyway, I had been following this channel for a long time and here is one similar demonostration like yours which I think is quite impressive as yours too ~ Nice work and enjoy :D