Glowing Rocks, Diamond Rain, Navigation, Ultra Violet Tendencies_ ..-....-.. __ ..-....-.. _Interesting Geology!
Learn surprising facts about some of my favourite Geologic Specimens. Quartz, Diamond, Iceland Spar, and Sodalite.

Image Credit:- wikimedia
Introduction
Hello everyone, today I decided to write an article about a few types of minerals. The study of Geology is very vast with many sub-branches, it's a truly intriguing area of study. This article will focus of some cool properties and interesting facts related to the four minerals I will talk about today, which are:-
- Quartz - Glass like silicate mineral, used for it's piezo electric response in lighters.
- Diamond - A carbon based mineral, extremely hard and not so rare as you may think.
- Iceland Spar - An old tool used by vikings to help navigate and find the Sun on a cloudy day.
- Sodalite - One of the many minerals that exhibits brilliant ultra-violet fluorescence.
I hope with the material I present I can teach you some interesting scientific facts associated with these minerals. I will leave references at the end of this article, they will lead you to resources where you can study the topics further if you wish.
Quartz

Image Credit:- wikimedia
Quartz is a silicate oxide, it's chemical formula is SiO2. This molecule is the repeating cell unit which leads to a tetrahedral lattice, which gives rise to the hexahedreal (6 sided) large structure of the crystal. Crystal grow from the atomic level to a large scale which you can see, every crystal is individual and unique. As the crystal grows under great heat and pressure they are impurities contained within silica oxide, such as iron. This creates something called dislocations where the lattice structure is deformed which means the crystal structure is not repeating through the entire specimen. Eventually these dislocations can lead to new crystal branches as you can see in the image.
Different types
These are lots of different types of quartz crystals that are given different names. Amethyst is also Si02, but contains a larger amount of iron that "pure" Quartz, this iron changes the atomic transition energy level, which lead to a purple colour. Rose quartz is a nice pink-reddish colour, its also silicate but contains impurities of Titanium, Iron, or Manganese. When these impurities form bonds with the Silicon and Oxygen in a the crystal lattice it creates a bond that has a characteristic colour of light associated with the energy in the bonding. White light of many frequencies hits the crystal and some of the light is exactly right to excite the atoms in the crystal. After being excited they must relax and by doing so they emit a specific colour of light, in fact this is the same process for every material.
Along with the many types of different quartz varieties, you may also find quartz and gold in the same location. Gold veins are usually found in areas where the quartz content is high. In fact it is a main clue to gold miners and was a valuable bit of information to know before modern day technology.
Triboluminescense
Another interesting feature of Quartz is its triboluminescent properties. I have written an article you can see HERE. Basically light is produced when the chemical bonds in the lattice are broken. The chemical bonds in the lattice release energy when they are broken, and it translates into a beautiful glow of the quartz. Check out my article to see a video of it in action, you'll be very surprised.
You can see another example Here on Wiki
Piezoelectricity
An important and useful property of Quartz is it's piezoelectric response. The piezoelectric effect is the ability of crystal to produce a voltage when the lattice is deformed through pressure. If you apply pressure to the bonds in a crystal they produce a voltage, and this also works in the opposite sense. If you apply a voltage to the crystal is deforms the crystal lattice (not breaking it). This is very useful, and has been implemented in many devices, the simplest in the electric lighter. You apply pressure to the crystal when you push on the button, at a critical point the crystal react and produces a spark from the energy created as you apply pressure, that spark ignites the gas.
Ultra-sound scans also use this effect, they apply a fast alternating voltage on a quartz crystal and it oscillates at the frequency of the AC applied. The crystal vibrates and causes ultra sonic sound waves, when they reflect back to the crystal it resonates it producing a voltage. This is then measured and used to create images based on the attenuation and acoustic impedance.
They are numerous implementations of this effect, and you can find many of them in the reference provided.
Diamonds

Image Credit:- wikimedia
Diamond structure
Most people will be familiar with diamond, it's made from Carbon, but there are other materials we know made from carbon like graphite in pencils. What makes them different is the bonding between the carbon atoms. A carbon atom has 4 electrons in its outer-shell, a full outer-shell would need 8 electrons, so keep it in mind. In graphite one carbon atom is covalently bonded (shares electrons) with another 3 carbon-atoms as you can see in the image.
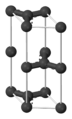
Image credit:- wikimedia
Graphite is a layered, planar structure, with individual layers are called graphene, it consists of 3 covalent bonds with the potential for one more. Diamond however makes the most out of the four opportunities to create covalent bonds with other 4 carbon-atoms, this is what makes it so strong and results in the characteristic appearance. Diamond has an active atomic transition that occurs in the deep UV spectrum at about 225 nano meters, so it is able to transmit visible light with no absorption effect, giving it the clear appearance. However if you look at the wavelength of 225 nano meters you'd be quite surprised. The strong bonding means that diamond is the hardest naturally occurring mineral in the Mohs scale, it's used in some hardware tools such as saws to help cut tough metals.
Trying to melt a Diamond?
Some interesting facts about diamonds now. Most materials go through different states of matter when the temperature is changed, ice to water to steam. However, this is not the case for diamond. If you tried heating up a diamond, in normal conditions the diamond would burn like coal exposed to oxygen at temperatures of about 3500 degree. If you put the diamond in an oxygen free environment and heated it to near 4000 degrees it would turn from solid to vapour without turning into liquid. Please if you have a diamond don't try to heat it up, I'm not responsible for expensive experimental mistakes haha.
Star Sized Diamonds
When a normal sized star finishes it's main phase, it becomes a Red Giant, after losing the outer shell of hydrogen all that remains is a White Dwarf. I don't think it should be called a White Dwarf, I think it should be called A WHOPPING HUGE DIAMOND! A star like the Sun goes through the process of fusion, hydrogen to helium, to lithium and boron, and then creates carbon (there's more to it than that). The Carbon is highly compressed due to the huge gravity the star possesses, which forces the carbon to arrange in the most energetically favoured state of being Diamond like. As the white dwarf cools over billions of years it leaves a brown dwarf that consists highly of carbon and is like a big diamond. So next time you think of buying a diamond ring, just remember its probably one of the most common materials in the Universe.
Diamond Rain

Image Credit:-wikimedia
There is strong evidence to suggest that on the Gas-Giant of our Solar system diamonds are formed in the highly pressurised atmosphere. Planets like Uranus have a lot of methane in the atmosphere. A study found that something called shock-wave compression can separate the carbon from hydrogen and the pressure cause the carbon atoms to bond together as diamonds, and rain down to the surface. Studies strongly suggest that under the thick atmospheres of the Gas-Giants are diamonds beyond your wildest dreams. Nature can do fascinating things right?
Iceland Spar

Image Credit:-wikimedia
This mineral is a type of calcite, more specifically Calcium Carbonate - CaCO3. It's a relatively soft crystal and can be scratched with the finger nail. It has a triagonal crystal structure, and is usually colourless, white or grey. It it a crystal native to the Iceland and is believed to have been used for navigational purposes by the Vikings. It demonstrates high polarizability of light (letting light through in a certain direction) and something called birefringence, which is double refraction of light. If you draw and image on some paper then take this crystal and start rotating it, at some point you will see a double image, as demonstrated in the GIF below.

Image Credit:-wikimedia
The Viking apparently used this Sun stone to find the location of the Sun on a cloudy day. In the Artic the polarisation of the Sun light can be detected, and using this stone it's possible to tell the position of the Sun in the sky with a small error of a few degree.
I really like this crystal due to it's birefringence properties, after studying birefringence you can understand te beauty of this effect.
Sodalite - Ultra Violet Fluorescence

Image Credit:-wikimedia
Sodalite has a chemical composition of Na8(Al6-Si6-O24)Cl2, and is one of many minerals that display the phenomena of fluorescence, when exposed to light specifically in the Ultra-violet range (100-400 nm) of the EM-spectrum. The Sodalite is usually blue with white vein, but can also appear grey, yellow, green, or pink. The Crystal grow with cubic geometry, as you can see in the image it's very cubey. When you shine UV light on these rocks they immediately change their appearance, from boring blue to an outstanding orange.
The UV light excites an atomic transition of the sodalite, and when the excitation decays the light emitted is in the visible spectrum as you can see in the image. The light emitted is nearly monochromatic (1-colour) with a small range of frequencies. In this case the energy absorbed of UV light is more than the energy of the light emitted, this is the basic concept of fluorescence.
Sodalite is just one example but there are many many examples, I have created a link to a list of in the reference section below.
Just take a look at this outstanding image for a moment, but it's 100 times better in real life believe me.

Image Credit:- wikimedia
Fluorescent materials are different to phosphorescent materials. After the Uv light starts shining the UV fluorescent effects stops immediately, but phosphorescent continue to glow like the bedroom stars due to long relaxation times of atomic transitions.
Fluorescence is the result of an atom or molecule relaxing to the ground state by emitting a photon of light from an excited atomic singlet state, the governing equation for absorption and excitation is:
The fluorescent emission is governed by the equation
S0 and S1 are the energy level states. The ground energy level S0 is excited with some UV photon and goes to the energy level S1. S1 then decays back to S0, but this time the light is emitted with less energy and the remaining energy is heat. I hope you can understand that concept, of course the physics behind this is quite deep, but I'm sure know you see how it works.
Some mineral hunters actually go to areas of known UV minerals at night and hunt for the specimens with a UV torch. Without the torch you would have no idea this process was occurring, unless you were a bumble bee that can see UV light.
Conclusion
So today I tried to tell you a lot of neat things that I hope you found fascinating and really caught your attention. We looked at Quartz and it's properties of being strongly piezoelectric and triboluminescent, and the different types you can find. Diamonds are a lovely mineral, not so rare as you think, practically everywhere from dead stars to rain on other planets. Iceland Spar is a type of calcite that can be used for navigational purposes due to it's polarising properties, and it has a peculiar property of birefringence. Finally we introduced Sodalite, a mineral known for it UV fluorescent properties, we looked at the science of it and hopefully you can understand.
I would really like your feedback, thoughts, comments, do you have anything cool to add? Geology is a vast subject with many surprising processes occurring in this field of study. I hope you enjoyed everyone, until next time.
Physics.Benjamin
If you liked this post feel free to UPVOTE, FOLLOW, and RESTEEM.
References:-
Quartz - Wiki
Triboluminescence - Article
Piezoelectricity
Diamond - wiki
Diamond - gia.edu
Extra-terrestrial Diamonds
Birefringence
Iceland Spar
Fluorescence
List of Luminescent Minerals:-Here
All images are Creative Commons or public domain, no copyright infringements have occurred.


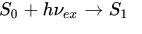
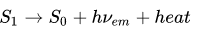

You received a 10.0% upvote since you are not yet a member of geopolis and wrote in the category of "geopolis".
To read more about us and what we do, click here.
https://steemit.com/geopolis/@geopolis/geopolis-the-community-for-global-sciences-update-4
Thank you :D
Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 14 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 20 SBD worth and should receive 73 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePigOn a similar topic, I recommend to read the series of posts written by @muphy called 'diamonds in the sky'. It is really very good! :)
Thank you, I'll take a look at it.
We have a fluorescent mineral room in our store. Only long wave at this time. We are searching for an appropriate short wave light at a reasonable price to set up an alternating long and short wave display. Cool specimens.
Yeah very spectacular once activated. I once ran a workshop with the museum about different minerals, but we only had long wave. It would be cool searching for them at night :)
@steemstem thank you for the upvote and recognition. I'm glad you enjoyed the article.