Pig Liver Esterase: A powerful little Friend of Biochemists
Today I can again provide an interesting insight into my daily work, which arises as part of my current research. I would like to introduce you to one of my much beloved enzymes, the:
Pig Liver Esterase (PLE)
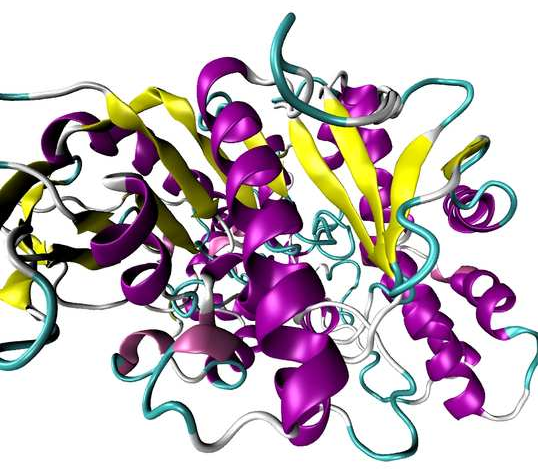
Structure of a PLE enzyme - Source: Ref.1
α-helices, β-strands and random coils are indicated in magenta, yellow and cyan, respectively.
Esterase enzymes are formed in almost all living organisms and thus are ubiquitous. These enzymes specifically cleave ester bonds and thereby form carboxylic acids and alcohols. This reaction is also called 'saponification'.
However, in my particular case, I use the PLE not only to cleave a carboxylic acid ester but since the intermediate formed is a β-keto carboxylic acid, another typical reaction step occurs: a decarboxylation.
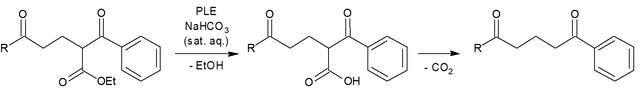
Since esterases cleave single bonds in a reversible fashion, these enzymes are classified according to the international EC Classification as Hydrolases.

Reaction buffer with yellow oily ester.
Image taken with Honor 5C Smartphone
For my biotransformation, the yellowish β-ketoester together with the aqueous sodium bicarbonate buffer are introduced into a reaction vessel. Since the ester is quite non-polar, it does not mix well with the aqueous buffer. Shaking the vessel gives a turbid, yellow suspension.
Next, only a few milligrams of PLE are added, and the septum integrated in the cap of the container is penetrated with a syringe. This measure allows the evolving Carbon dioxide gas to exit the container.
It counteracts both a potentially dangerous overpressure in the reaction vessel, and also the equilibrium of the reaction is favorably drawn towards product formation - see Le Chatelier's principle.

Esterase from porcine liver.
Image taken with Honor 5C Smartphone
And Yes: In fact, the enzyme used here got that name, because it is extracted from pig livers. This is both the most economical and ecological production route for this enzyme, which is also relevant in industry.
Next, the reaction mixture is shaken at a temperature, that is ideal for the enzyme activity and and also its stability: 30°C for 24 h. Thank goodness we again can make use of helpers, who do this work without grumbling and who we can even adjust to an exact shaking frequency:

Shaker set to various temperatures and shaking speeds.
Image taken with Honor 5C Smartphone
Finally, the workup of the reaction mixture has to be done. This is the part that normally represents the greatest work for the chemist himself. But with this experimental approach, even the work-up is quite manageable:
- The reaction mixture is treated with hydrochloric acid.
- Multiple extractions with ethyl acetate are performed.
- The new organic phase containing the product is dried with MgSO4.
- The dry organic phase is transferred to a final product container.
- The solvent is removed by using a so called Rotavapor.
The magic is done and I got my product in almost perfectly pure form!
mountain.phil28
References:
- Hasenpusch D. et al. J. Mol. Model., 2011, 17 (6), pp. 1493-1506
Being A SteemStem Member
btw I was curious why dont do a basic ester hydrolysis? I assume the decarboxylation is pretty readily take place under such condition(?)
or actually you are intentionally investigating the enzymatic transformation?
We have lots of lipase in our lab. Let me see if I can find a picture for it XDD
What's wrong with my Intro? 😋
Yes, the decarboxylation ist indeed rather spontaneous. We use the enzyme to perform a soft ester hydroylsis in order not to damage oder unintendedly transform other parts of our substrate. ;-)
Label your molecule with this 🤣🤣 (i used to walk on egg shells too)

I guess it will be hard to print such tiny warning signs. HAHA! :)
Nice transformation, I'm having trouble thinking of another way to cleave the ethyl ester in one step.
PLE is also commonly used to react with only one enantiomer in order to allow for purification of racemic mixtures right?
Yess it's possible to use these enzymes for (kinetic) enantiomeric resolution.
GL with your lab-problem. ;-)