Surviving the Extremes: The Deep, Part 2 - The Revenge of the Snailfish
In my previous post, I looked at the incredible ability for some animals to dive deep down into the darkness of the oceans without their lungs imploding and their ears melting, which is kind of what would happen if you went 10,000 feet down like some of the whales do.
But these guys are still limited to the depth they can go, because they still have air within them, and water is heavier than air. For a bit of perspective:
...one atmosphere equals about 14.6 pounds per square inch pressure, and the pressure increases 1 atmosphere for every 10 meters of depth. Source
The deepest fish known, that I could find anyway, is the snailfish, 8,145 metres below the sea at the Marianas Trench.
With a little maths and conversion, I work that out to be 5,393kg per square inch of pressure. A snailfish can reach about 30 inches in length, and judging by its appearance, I'd say roughly 7 inches on a side. If we deconstruct the fish into a basic rectangle, that's 2102 inches.
This means that, very roughly, the poor snailfish has to endure 1,132,530kg of pressure pushing against its body.
This is equal to 416 full grown African Elephant bulls sitting on top of this tiny deep sea dweller! Here's a picture I made to help you out:

Not to scale
But... it doesn't quite work like that.
I explain in part about atmospheric pressure (properly called hydrostatic pressure when underwater) in part one so you can get a more realistic idea. But basically, it's pressure difference rather than absolute pressure.
The cold, solidifying fats
The deeper you go, the harder it gets to live, no matter what. Animals thriving down at the depths of the snailfish do away with any kind of air pockets whatsoever; think squids. Their body is solid and their blood is liquid. Both of these forms of matter don't really compress at all, water perhaps 5-6% compression at the deepest points on earth. So the liquid blood and goo in a squid is basically unaffected, allowing it to swim around in all its gelatinous glory.
(Some industries like to substantially compress water and shoot it out at 600mph, slicing through stone and even metal. Remember the poor snailfish enduring 5,393kg of pressure per square inch? Well, this industry requires at least 40,000kg of pressure, so it's not really applicable in the natural world.)
But beyond the squid and snailfish, some new problems arrive. The cold mixed with the super-massive-extreme pressures cause even the fats of the cell membranes to solidify. I dunno about you, but I wouldn't want my blood cells hardening into solid blocks. If nothing else, this crushes the proteins within the cells, making them, well, dead.
To get around the coldness freezing them (note; the sun can't penetrate past just a few hundred metres below the sea), they've managed to surround their cell membranes with unsaturated hydrocarbon fats which lacks water, thus freeze at much lower temperatures.
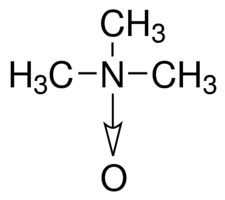
As for the pressure, evolution really had to think about this one. The theory goes that the animals collect piezolytes, a molecule that binds tightly with water, allowing more space around proteins, preventing them being crushed. One piezolyte, called TMAO, is the molecule responsible for the fishy smell of fish we all know and hate. Well, more piezolytes are needed the deeper you go, and sure enough, more TMAO can be found in deeper fish. I wouldn't want to be up close with those fish, let's put it that way.
HOWEVER
The piezolytes theory has been called into question and the whole theory has been thrown into doubt very recently. The paper you can read below only came out in July this year.
So even at this point in the ocean deep, say, 7000 metres or so, the actual method of survival is still kind of a mystery. Nonetheless, by about 8,000 metres, fish cease to exist. That we know of...
Building Sandcastles
11,000 metres down at the bottom of the deepest parts of the ocean lie xenophyophores. These are unicellular lifeforms with a neat little trick to get through the day (if you can call it that. Which you can't).
You see, a shell would be a nice form of protection down there, a roof over your head from all that weight, but your typical calcium carbonate shell fails miserably at this; the crazy pressure simply dissolves it.
By collecting sand and gluing it together with old skeleton parts and even bits of its own fecal matter, a xenophyophore has found a material that can in fact withstand the crazy pressures and thus a way to survive, pressure-free.
Don't imagine them to have some rock-hard protective turtle shell thing going on though. They're actually so delicately structured that they cannot be lifted from such depths for study, they inevitably fall apart.
These, along with the rest of their family, foraminifera, have more or less dominated the oceans around the world, finding their niche unchallenged by anybody else.
Except giant Shrimp and Sea cucumbers. And microbes.
But to be honest at this point, we still don't really have a clue how they're there, and for more reasons than pressure alone. There are so many other factors that should have, by any logic, killed off all life down there.
For the plethora of microbes, it seems to be the case that they're surviving on the ambient temperatures of chemical reactions down at the sea floor between the rocks and the water, while feeding off hydrogen and methane those rocks release.
BUT, that is a whole other story for part 3! Don't hold your breath though (seriously), the answers from this point out are largely:
We don't know

Image sources cc0 licensed (or made by me)
Sources
BBC
Scienceabc
Fiboni
Scientific American
Piezolyte Doubt (login required, sorry! Abstract available)

Should we go together throw elephants into the water to smash a couple of snailfish? What do you think?
You deserve such a comment with your elephant picture.
Oh sorry I already did it! The elephant picture is an actual photo, in case you didn't realize... =P
I looked to the picture too fast... Am guilty! :p
I definitely don't know enough about animals under pressure. I seriously thank you for the piezolyte note. Although it was a weak hypothesis I was unaware of this article.
There's too much sh*t to read to keep up to date.
Same for me. I know much more about human beings under pressure (but not the same pressure) ;)
This post was upvoted & promoted by @monitorcap traffic bot.
Send minimum 1 SBD to @monitorcap bot with your link in MEMO field and recieve random upvote & post promotion in our daily TOP posts listings. @monitorcap - where 'seen' matters !
Despite the final 3 words, I learned a few things, some of them even applicable to cooking (when it comes to choosing fish, the surfacer the better)
unless that July paper is right
Well, even if the latest paper is right, it doesn't disassociate TMAO with flavour, just that it's not a necessary component of deep sea existence, and also, it's not applicable to cooking anyway since all sea food is gross.
Except Tuna, which clearly isn't a real fish.
I'm pulling my non-existent hair right now!
Have you tried simple fried fish with olive oil-lemon drizzle like they do in most Italian and Greek seafood restaurants? You just find fish yucky in general?
Just the common theme smell, flavour and often texture, just gross. I also hate Olives. Greece just isn't for me I guess! =P
Beutiful picture
Beautiful view